We live in close contact with bacteria, viruses and fungi. Some of them are even part of our microbiome. However, not all micro-organisms are harmless and there are pathogens that cause infections and diseases. When a pathogen enters the body, it triggers the activation of several complex processes aimed at eradicating the infection.
Attack and defence. An action drama in three acts.
When a micro-organism enters the body, several defence mechanisms are activated. To protect our bodies, we need:
- A system capable of containing the infection in its early stages.
- A system capable of completely eradicating the infection.
- A memory to remember which germ has infected us and to fight it more quickly in the future.
Act I: Speed to contain the infection
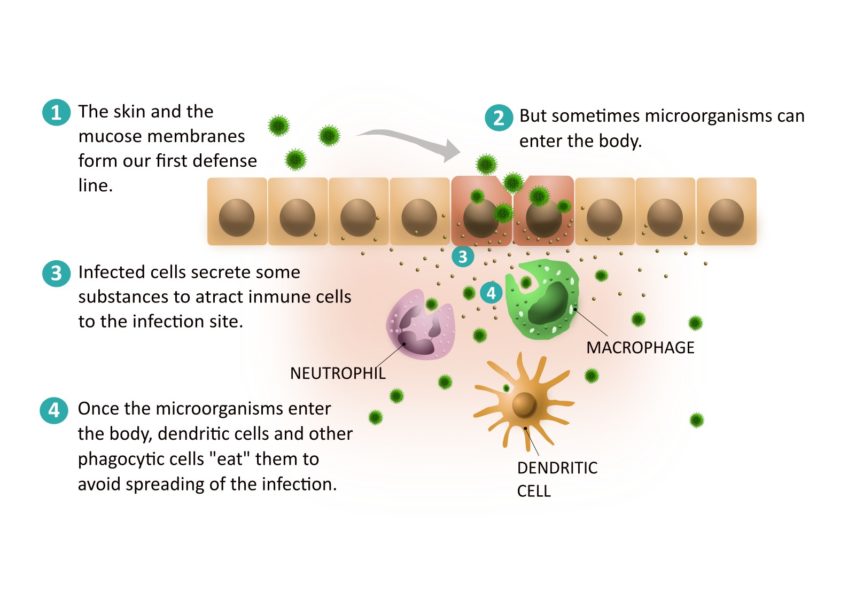
The first defence to be activated is called the innate immune system. Our skin, together with the mucous membranes and inner membranes of the digestive, respiratory and urogenital tracts, form our first line of defence. In addition, secretions such as saliva, tears and mucus contain substances that destroy microorganisms. However, some pathogens manage to get past these physical and chemical barriers and enter the body. At this point, other components of the immune system are activated. They help us fight the infection, but how?
First, the infected cell secretes substances called interferons that protect surrounding cells from infection. In this way, it tries to protect these other cells and prevent the infection from spreading. At the same time, these substances act as a ‘flag’ for other immune cells to migrate to the site of infection.
The first immune cells that will try to contain the infection are already present in the inner layers of the skin and mucous membranes. These are dendritic cells and other phagocytic cells. They recognise proteins and structures that are present in bacteria, viruses and fungi but not in our cells. This triggers a rapid and non-specific response, meaning that they react similarly to different pathogens. This response occurs during the first few days of infection, helping to control the infection and allowing time for specific mechanisms to be activated.
Dendritic cells and other phagocytic cells in infected tissues are able to ‘eat’ the microbe causing the infection. Once digested, the dendritic cells travel to the lymph nodes to activate the cells that will mount a more specific response to eradicate the infection: the cells of the adaptive immune system.
Act II. Specificity to eradicate infection
So how is the adaptive immune system activated?
The cells that give a specific response are both B and T cells (also called B and T lymphocytes). In the absence of an infectious agent, these cells circulate as dormant cells throughout the body. Each B-cell and T-cell carries a unique receptor, which is made at random. Each receptor is specific and can recognise a foreign molecule called an antigen. In the absence of these antigens, B and T cells remain inactive.
T and B cells occupy different locations within a lymph node. When a dendritic cell arrives, it meets inactive T cells. Dendritic cells that have processed the microbe present small pieces of microbial proteins (peptides) to the T cells. This presentation is made by molecules called HLA molecules. If a T-cell expresses a specific receptor that can recognise the HLA-peptide complex: bingo! The cell with the “winning” receptor undergoes clonal expansion: it becomes activated and starts proliferating, forming an effector T-cell clone. Unlike inactive T cells, effector T cells can be activated to help fight infection.
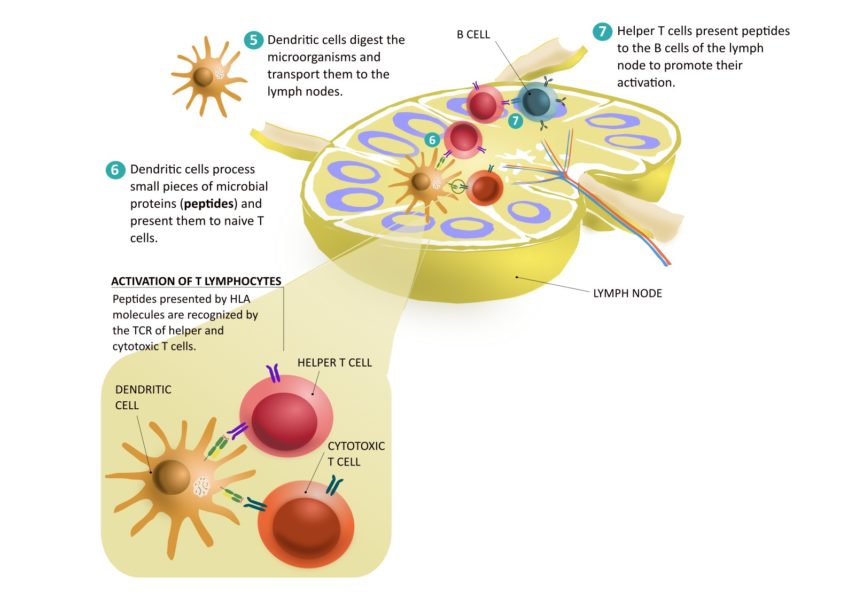
There are two types of effector T lymphocytes: cytotoxic and helper T cells:
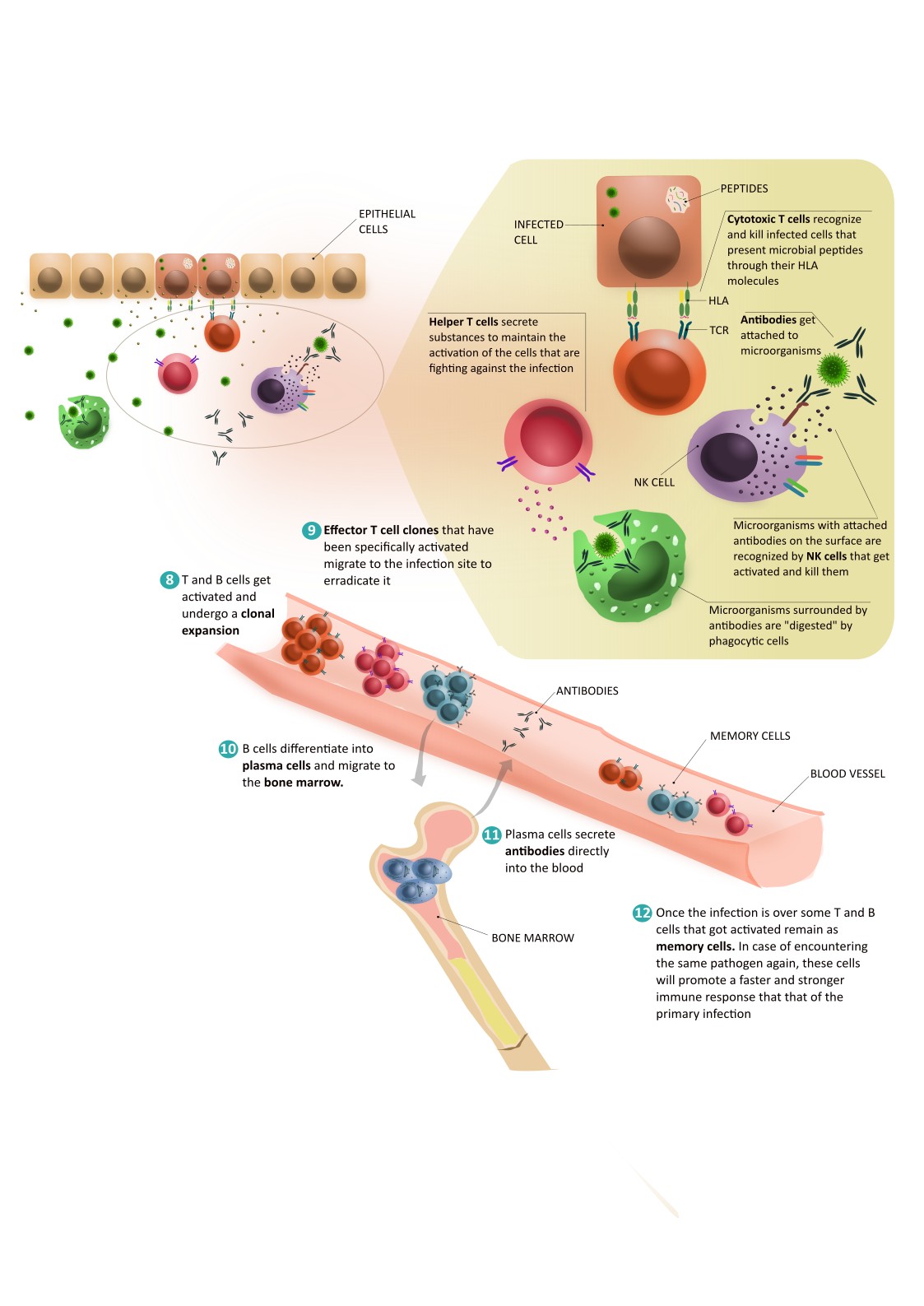
- Cytotoxic T cells leave the lymph nodes and travel to the site of infection. Once there, they receive signals to leave the blood vessels and enter the tissue. There, like dendritic cells, they recognise infected cells that present pathogen peptides through their HLA molecules. Cytotoxic T lymphocytes recognise these peptides and kill the infected cells.
- Helper T cells secrete substances to maintain the activation of cells involved in the defence against infection. Helper T cells are also involved in the activation of B cells in the lymph nodes.
So we know how T cells are activated and what they do, but what about B cells? B lymphocytes are activated by interaction with helper T cells. Activation of B cells promotes their differentiation into plasma cells. Some of these cells migrate into the bone marrow. From there, they secrete antibodies directly into the blood. These antibodies protect us from pathogens. They have different mechanisms of action. Among other things, they:
- Block the entry of germs into cells.
- Attach themselves to microbes to mark them so that these can be ‘eaten’ by innate immune cells.
- Attach themselves to infected cells to mark them for killing by NK cells, a type of innate immune cells capable of recognising antibodies.
Plasma cells can produce antibodies even decades after exposure to the pathogen. After infection, they continue to secrete antibodies at low doses. In this way, we are prepared for a new attack by the same micro-organism.
Act III. Memory to remember the infection
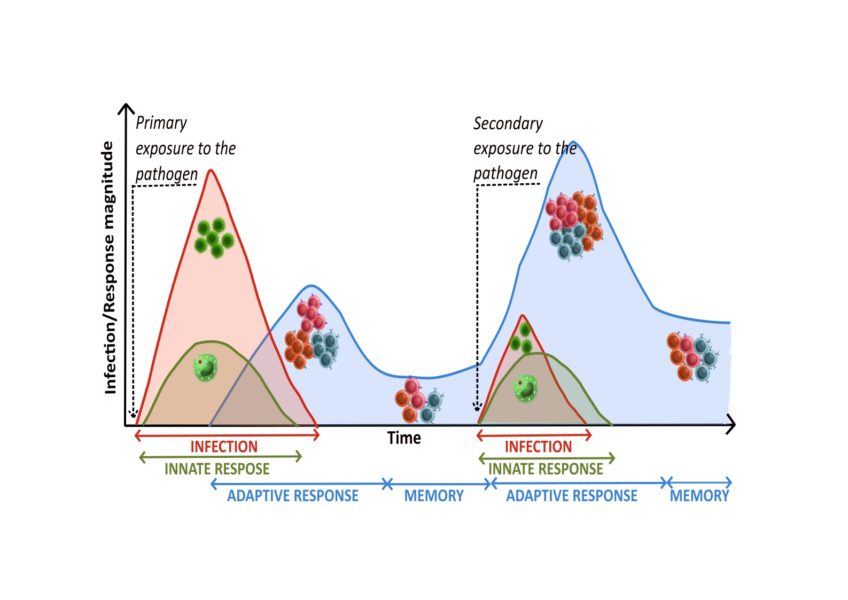
After the infection has been fought, some of the specific T and B cells produced remain as memory cells. These cells travel around the body, retaining their ability to recognise the antigen by which they were selected. When they recognise cells that have been re-infected by the same pathogen, they are ready to be activated, to divide and to do their job. This promotes a faster and more powerful response to secondary exposure to the microbe than to primary exposure.
Vaccines help us generate memory cells before we are infected by the pathogen. These are generated using pathogen fragments, pathogen inactivation and other strategies to stimulate the immune system to simulate the response that would occur before a real infection. In this way, we create an immunological memory that will quickly protect us in the event of infection with the real pathogen.
The microbes’ counter-attack
While our body is fighting the infection, the microbes are not sitting still! For each type of infection, there is an immune response that has evolved to match the specificity of each germ. At the same time, pathogens have been selected that are able to escape and evade the action of the immune system. This is the case with some viruses.
There are some viruses that have evolved to trick our defences by interfering with the recognition of infected cells. One of the mechanisms they use is to promote the downregulation of the expression of HLA molecules. In the absence of HLA molecules on the cell surface, infected cells cannot present viral peptides. Therefore, T cells cannot recognise them as infected and cannot kill them.
So why aren’t these viruses more dangerous? Because we have other defence mechanisms besides the ones we have talked about. For example, NK cells (natural killer cells) are a type of lymphocyte that are crucial in the defence against viral infections. NK cells are innate immune cells. Under normal conditions, these cells are suppressed. However, during a viral infection, they can sense the lack of HLA on the infected cells. They then secrete substances to kill them. This is an example of how complex the immune response is and how it has evolved to fight each type of infection.
The immune system and pathogens have evolved together. In some cases, they have reached an equilibrium. This is the case with latent infections. The immune system controls the infection but cannot eradicate it completely, and at the same time the microbe can survive in the body in a ‘dormant’ state. Examples of these are cytomegalovirus infections or infections caused by tuberculosis bacteria.
It is important to study how we protect ourselves against infectious microorganisms and to understand how microbes respond to our immune system. This will help us understand how infections work and what therapeutic strategies can be used to combat them. At the Pompeu Fabra University Immunology Group, led by Miguel López-Botet, we are studying the changes that cytomegalovirus infection induces in our NK and T cell compartments. We are also studying the immune response triggered by both NK and T cells prior to viral infection.