ALLOX is a spin-off of the Centre for Genomic Regulation (CRG) in Barcelona, founded by researchers Ben Lehner, Júlia Domingo, Pablo Baeza, and André Faure. It is a techbio company building the next generation of tools to predict and design new drugs based on allosteric regulation.
In this interview, Júlia Domingo, CEO of Allox, tells us how the company was born at the CRG, how the technology they developed works, and what its future holds.
Júlia is a biologist, bioinformatician, and co-inventor of this pioneering technology. “I am proud to realize the revolutionary potential of our technology and to test the basic thesis of our research in the clinic while leading and growing with such a stellar team”, she says.
“I am proud to realize the revolutionary potential of our technology and to test the basic thesis of our research in the clinic while leading and growing with such a stellar team”
Júlia Domingo, CEO of ALLOX, a CRG spin-off
How was ALLOX created?
ALLOX originated in Ben Lehner‘s lab, where the four founding partners met. Pablo Baeza and I were doing our PhD, and André Faure did his postdoc and later became the lab’s Staff Scientist. In the second part of my PhD, we fine-tuned ALLOX’s technology. We published it in a scientific paper in 2022, which piqued the interest of pharmaceutical companies and venture risk investors. This motivated us to make the leap to a company. Although we came from basic research, we saw that what we had created had a lot of potential for application.
We started setting up ALLOX towards the end of 2022. We were all working in various labs in different countries (I was in the US) and developed it in our spare time. I created the experimental part of this technology, and André Faure developed the computational part. Pablo Baeza brought his data processing experience. Ben Lehner has guided us through this process. An important factor is that the team understands and works very well together. Formally, ALLOX began in November 2023 and has been operational since the end of January this year (2024).
Did you imagine you would be the CEO of a start-up when you started doing research?
Honestly, I never considered starting a company. I always thought I would pursue an academic career and was even reluctant about the business world. But I have always been clear that I want to do cutting-edge, innovative, robust, and fair science. Additionally, I enjoy mentoring and wanted to lead a team.
Living in New York during my postdoctoral studies helped me realise that one can do all of this in a startup. The city boasts a thriving biotech startup ecosystem, and I was fortunate to be surrounded by scientists either launching their own biotech ventures or transitioning from research to the biotech world. This immersion in the industry completely reshaped my perception of this world.
There is another important reason to consider coming back and setting up the company. I’ve always believed the science we did at CRG was cutting-edge with immense potential for understanding biology at scale. However, by building a company around this technology, we could have a real impact in the clinic, potentially improving millions of lives.
The number of women CEOs in tech companies is growing, but you are still only 15% in Spain…
Yes. It is clear that women are much less represented in executive positions, not only in the biotech sector. Things are improving, but we still have a long way to go. This also happens in science, where there were far fewer women as group leaders, and there are more and more now.
“It is evident that women are much less represented in executive positions, not only in the biotech sector. The situation is improving, but we still have a long way to go.”
I think what helps a lot is the visibility of women in these positions, having role models. Grants or scholarships that promote equality in executive positions or fund women-led companies, like those from the European Innovation Council (EIC), are increasing, but more are needed.
Another thing that needs to change is parity in other areas closely linked to business, such as investment funds. Biotech start-ups don’t generate profits initially and need investors. It is proven that women-led companies receive less investment. Therefore, we need more women leading investment funds and for the investors’ mindset to change.
About your technology: you talk about the “holy grail in drug development” and a methodology that allows you to find “a needle in a haystack”. What do you mean?
Our technology allows us to find allosteric sites in human proteins, which are very interesting drug targets.
Proteins have an interaction surface that binds to other proteins to perform their function. They also have so-called allosteric sites, which are not found on the interaction surface. Other smaller chemical substances can bind to these allosteric sites to generate changes in the protein and regulate this interaction.
“Our technology allows us to find allosteric sites of human proteins, which are very interesting drug targets”
Allosteric sites are a target with a lot of potential for drug discovery. These sites tend to be much more specific to each protein because they evolve differently than the interaction interfaces, which are more evolutionarily conserved. This makes allosteric drugs more specific, targeting only the protein that needs regulation and reducing the likelyhood of toxic effects.
Until now, most allosteric drugs have been discovered by chance and there were no systematic ways to find these allosteric sites experimentally. At ALLOX, we can now create maps of allosteric sites like never before and at an unprecedented speed.
Can you explain how the technology you have created works?
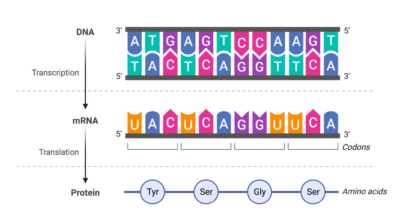
This technology allows us to systematically study all the sites of a human protein by experimentally altering each amino acid of a protein one by one and analyzing the consequences of these mutations on different molecular phenotypes. Additionally, we analyze these mutations in many different genetic contexts, for example, in the presence of other mutations. This approach enables us to see the effects of these mutations from multiple perspectives in one single experiment.
Our innovation lies in our ability to generate a large number of results in a short amount of time. Traditionally, proteins have been studied by making individual mutations and observing the outcomes, mimicking evolutionary processes. We do the same, but next-generation sequencing allows us not only to study a few select mutations, but instead to obtain data on all possible mutations and quantify their effects. We call this deep mutational scanning (DMS).
Since our experiments involve hundreds of thousands of protein variants in millions or billions of cells, we need computational approaches in order to process all these data. Using computers, we model the biophysical consequences of all these mutations in proteins, generating a map of the protein’s allosteric sites.
Your first goal was identifying allosteric switches in many proteins. Which proteins have you selected to begin with?
We have only been operational for six months, but we are very active and want to map as many proteins as possible. We have started with proteins lacking effective drug development or facing drug resistant issues, particularly those associated with cancer. While much is known about cancer-causing proteins, over 80% lack targeted therapies, and resistance to existing drugs is widespread.
“Our technology enables us to gather data on the biophysical consequences of mutations at an unprecedented speed”
Our technology enables us to gather data on the biophysical consequences of mutations at an unprecedented speed. In just 3 or 4 months, we can generate an amount of data comparable to all previously published data combined. This provides us with a unique opportunity to learn from high-quality data. Our ultimate goal is to build computational model that allows us to predict allosteric sites entirely in silico, without the need for experiments.
In a second phase, you want to develop new drugs. Are you close to this?
Yes, we believe that this year 2024 we will already start our first drug development program to generate new allosteric modulators that will culminate in innovative therapies for diseases that are currently untreatable. We are also considering partnering with other biotech or pharmaceutical companies that have experience in bringing these types of drugs to market.
There is a third phase to apply the technology in industrial, agricultural, and environmental biotechnology. Can you explain a bit more about that?
This is our horizon at ALLOX. The idea is that if we can accurately predict how changes to DNA influence protein function, we can start to engineer new biological molecules. For example, we could create proteins with switches that activate when certain substances are detected in the environment.
But engineering biology is much more complicated than civil or industrial engineering. We build cars very easily, but we don’t know how to build biological systems from scratch. One of the problems is that we don’t fully understand how all these biological components work. This is one of the goals of our research: to understand the relationship between DNA sequence and function.
How do you see ALLOX’s future?
We are very optimistic. The research team will grow, both in the experimental and computational areas. This will be the company’s most immediate task. New positions are now available, and larger laboratory space will be needed to accommodate the scaling up of our operations. Apart from research staff, we will need more team members to help us push the boundaries of our technology and support collaborations.
“There are not many laboratory spaces in Barcelona. This limits growth opportunities and discourages investments, often forcing companies to consider relocating.”
One of the challenges we see for Barcelona’s startups is that there are not many laboratory spaces in the city. This limits growth opportunities and discourages investments, often forcing companies to consider relocating. There is private investment in laboratory spaces, but more investment is needed, both private and public. We would like to be able to stay in Barcelona.