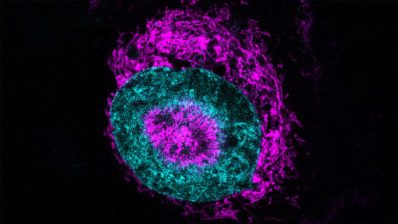
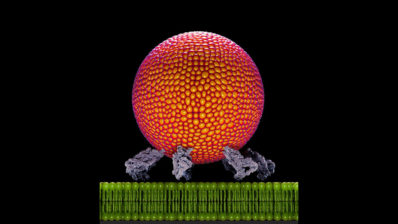
Researchers from the Department of Experimental and Health Sciences, Pompeu Fabra University (DCEXS-UPF) participated in an international study that has defined how tumor cells enter the bloodstream in the initial step of metastasis, a process called intravasation.
We have talked to Selma Serra, from the UPF Molecular Physiology laboratory specializing in ion channels, led by Miguel A. Valverde, to understand the importance of this finding.
What has been the role of your group in this work?
About 6 years ago, our laboratory contacted the laboratory led by Dr. Konstantinos Konstantopoulos to propose a collaboration. Our common interest was to identify the signaling molecules involved in the ability of cancer cells to become invasive and initiate metastatic processes.
We had hypothesized that some of these molecules had to be ionic channels, and the first experiments allowed us to discriminate which of these channels could be correlated with a greater invasiveness of the cells. We soon saw that the TRPM7 channel was the main candidate.
“When the TRPM7 channel was absent, cells dramatically changed their migration behavior”
Thanks to our extensive technical experience in this field, in addition to providing the experimental part, we have proposed key experiments that led us to describe the entire intracellular signaling pathway generated around the TRPM7 channel.
And how does the TRPM7 channel intervene in the intravasation process?
Our laboratory has spent many years studying ion channels, specifically those that are sensitive to osmotic changes or that are activated by mechanical stimuli such as the physical pressure exerted on the plasma membrane.
Thus, in our work we have shown that cells decide their course based on whether or not they are capable of detecting the shear forces exerted by the flow of fluid from the vessels they enter. This detection depends, to a large extent, on the TRPM7 channel’s presence at the plasma membrane of cells.
When the TRPM7 channel is present in the membrane and is activated by fluid forces, a signaling cascade is initiated and leads to a remodeling of the cytoskeleton. And this is how the cell that has been able to detect this fluid can rotate and escape from this mechanical stress.
“When a cell detects the fluid thanks to TRPM7, it remodels its cytoskeleton and avoids the mechanical stress”
We think that in cancer cells this mechanism is repressed and, therefore, with low levels of the channel in the membrane, the cells do not escape the blood stream, which facilitates the intravasation process, key to distant metastasis.
Do you think that these discoveries could be transferred to the clinic in the future? How?
More research still needs to be done, but the published results on the role of the TRPM7 channel in cancer are likely to promote an interest in clinical practice.
On the one hand, in terms of cancer diagnosis, it can provide valuable knowledge about the metastatic capacity of a tumor in correlation with the degree of expression of the TRPM7 channel or even possible genetic variants with loss or gain of function.
On the other hand, it could generate interest as a target molecule for the pharmacological treatment of cancer or even a gene therapy tool using the CRISP gene editing technique.
Christopher L. Yankaskas, Kaustav Bera, Konstantin Stoletov, Selma A. Serra, Julia Carrillo-Garcia, Soontorn Tuntithavornwat, Panagiotis Mistriotis, John D. Lewis, Miguel A. Valverde, Konstantinos Konstantopoulos. The fluid shear stress sensor TRPM7 regulates tumor cell intravasation. Science Advances 7 : eabh3457 9 July 2021.