Microtubules form both the skeleton and the motorways of our cells. Yet we still don’t understand how these essential structural elements are formed. New research by the Surrey lab — who recently arrived to the Centre for Genomic Regulation (CRG) from the Crick Institute — gives some light into how it all starts.
Microtubules are long tubes made of tubulin units (a and b) arranged in a circular, helix pattern. The formation of microtubules consists of two parts:
- Nucleation: the first a/b-tubulin units are arranged on top of a ring complex made of γ-tubulin units, called γTuRC.
- Elongation: more a/b-tubulin units are added to make the microtubules longer.
To understand how these processes are regulated, the team of scientists led by Thomas Surrey purified human γTuRC complexes and looked at the kinetics of microtubule formation in vitro.
What they found is that γTuRC is actually very inefficient regarding the first step. However, once the first units are arranged, the elongation step is much quicker.
γTuRC is actually very inefficient regarding nucleation, but once the first units are arranged, the elongation step is much quicker.
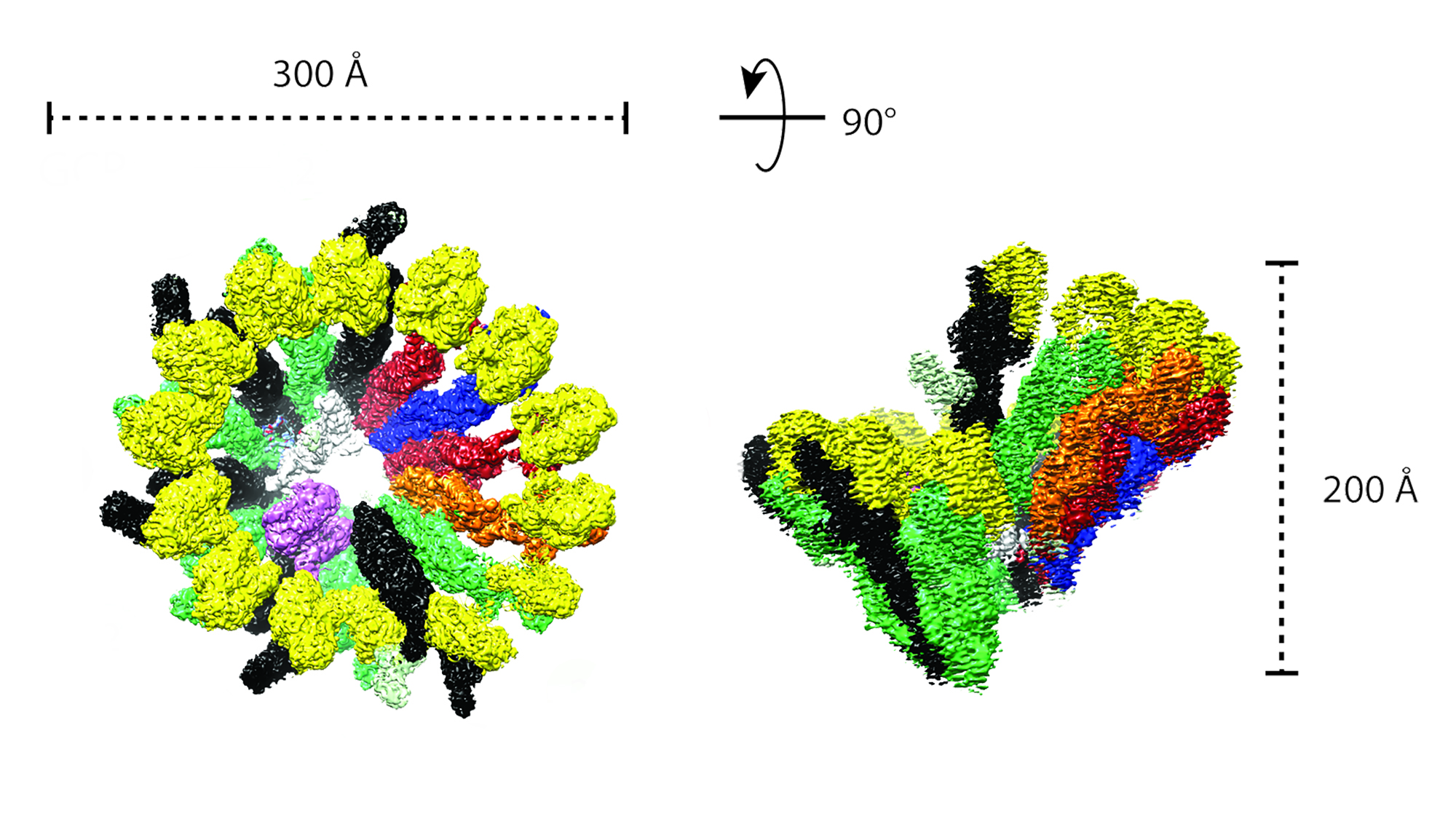
To decipher why γTuRC is so inneficient to begin with, they teamed up with the Alessandro Costa lab at the Crick who looked at the structure of this complex using cryo-electron microscopy — a technique which allows to see incredibly tiny structures (in this case up to 4A, or 4 billionth parts of a cm).
They found that, out of the 14 γ-tubulin subunits that form the γTuRC in a circle, half of them were close together in a compact configuration (closed) while the other half had gaps between them (open), making the whole ring asymmetric. This deviation from the microtubule geometry would make it trickier for the a/b-tubulin to jump in and start forming the microtubule.
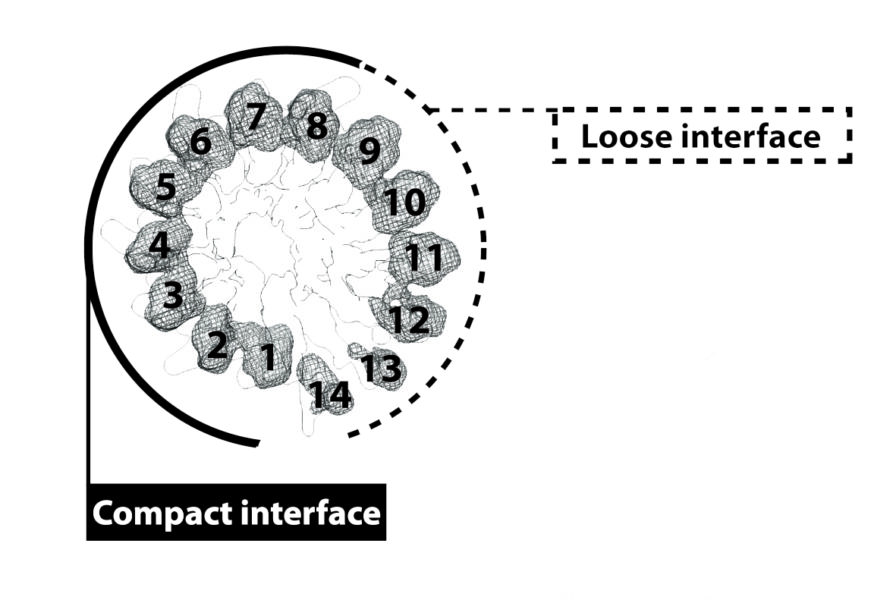
The scientists believe the way the γTuRC gets activated — and microtubule formation is sped up — is by a change of conformation of the ring, from this asymmetric open form to a more compact one, more similar to the actual microtubule pattern. The challenge now will be to find out which factors are involved in this change.
Tanja Consolati, Julia Locke, Johanna Roostalu, Juri Rappsilber, Alessandro Costa, Thomas Surrey. Microtubule nucleation properties of single human γTuRCs explained by their cryo-EM structure. Developmental Cell. Open Access. Published: May 19, 2020DOI:https://doi.org/10.1016/j.devcel.2020.04.019