About 95% of pancreatic cancer patients die within 5 years. The reasons for these bad results are a late diagnosis and inefficient treatment.
Two labs at the Department of Experimental and Health Sciences, Pompeu Fabra University (DCEXS-UPF) may have found a solution.
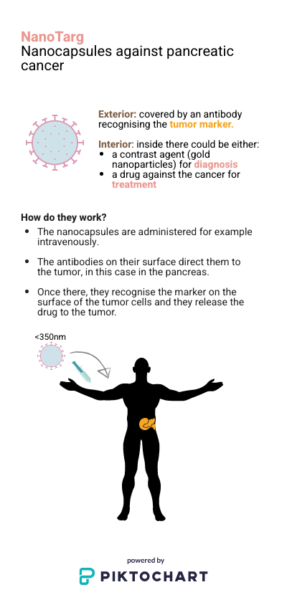
The teams led by Pilar Rivera and Rubén Vicente have developed a technological platform, called Nanotarg. It allows a pancreatic cancer drug to be encapsulated inside it while coating its exterior with specific antibodies against a tumor marker. This marker is expressed only in pancreatic tumor cells, therefore, the nanocapsule specifically targets the tumor, where it releases the drug. Consequently, side effects decrease and efficiency increases, since fewer doses are required to obtain the same results.
We talk to Rivera and Vicente, who are currently seeking further financing to continue the development of this platform.

How do these nanocapsules work?
Nanocapsules with a diameter of less than 350nm are coated with specific antibodies against a tumor marker that is overexpressed on the surface of pancreatic cancer cells. Therefore, after an intravenous administration, the nanocapsules go directly through the blood to the pancreatic tumor. As the drug is encapsulated, its side effects decrease. In addition, the elements that compose them are biodegradable, so they are assimilated by the body.
You say this technology is also useful for diagnosis. How does that work?
Nanocapsules also allow contrast agents such as gold nanoparticles to be encapsulated, rather than drug. Gold has a higher atomic weight and therefore gives better contrast than other agents currently used. Nanotarg therefore allows to diagnose lesions earlier because it accumulates large amounts of this contrast agent in the tumor thanks to the specific recognition of the tumor marker. It also allows metastasis to be detected, if an accumulation of nanocapsules is observed in other areas when doing the tomography.
Improving diagnosis is important because pancreatic cancer is sometimes mistaken for pancreatitis. In addition, being asymptomatic in the early stages, it is usually diagnosed very late, when there may already be metastases. That is why it is such a lethal disease.
Could this technology be useful for other types of cancer?
What defines our technology platform is the way to encapsulate and direct. In March we applied for a patent for this specific target of pancreas, in which we trust a lot for its very specific expression. But the marker we are working with seems to give tumorigenic properties and is also overexpressed in ovarian or lung cancer, so it could be useful in these, too; factors such as frequencies or when it is expressed should be studied.
What phase is Nanotarg in right now?
At the moment we have created a 350nm nanoparticle prototype specifically targeted at tumors and we have validated it in vitro (cell cultures) and in vivo (animal models). Indeed, we see that nanocapsules accumulate in tumors that express the specific target. The next step is to see whether in addition to concentrating on the tumor, the encapsulation of the drug achieves a better tumor reduction than the administration of the drugs without encapsulation.
“I am sure that the idea is very good, and that at some point it will be used. If we are the ones to come up with the final design to be used, it would be fantastic. But if not, at least we should advance as much as we can”
Rubén Vicente
When do you expect you will be able to use this platform in the clinic?
After functional validation, we still have to do toxicity studies, study how to produce the technology on a large scale, do clinical trials… It is a process that takes years! The concept may seem simple, but there are a thousand small modifications that can be made to optimize: the orientation of the antibody on the capsule, how the drug is encapsulated so that it is not lost along the way but is efficiently released, etc. As well as legal, industrial and regulatory aspects to comply with.
It all requires quite a lot of financing…
Yes! We hope to get the investment before moving on to the clinical phase. Until now we have had the support of UPF (Plan INNOValora, Maria de Maetzu), AGAUR (Product) and the Ministry (National Plan). But the next step to the clinic is very expensive and requires large capital investors.
So we seek funding from investment groups and high-risk capital, but also crowdfunding by patient organizations and foundations, among others, that see in our technology something that is worth contributing to and giving a boost. We have already passed filters from different experts in the field at the University, Generalitat, and Ministry levels, so we believe it is a relatively safe bet. We are people with desire, with a very clear idea, with experience in our fields and very rigorous!
“Nanotarg has all the potential to become a multifunctional product for cancer management, both its treatment and its diagnosis. The data indicates that our value proposition is valid and has the capacity to finish the pre-clinical phase to enter the clinic and be able to reach the market. If we succeed, the survival rate and quality of life of the patients would improve considerably”
Pilar Rivera
What has the technology transfer experience been like?
As scientists we are normally focused on our research and we are not familiar with the tech transfer environment… That is why we would like to highlight the support of the transfer team and UPF Ventures, who have helped us a lot to understand what the investors want, to learn how to present the selling points of our project… We have also received a lot of help since the beginning from the researcher Pilar Navarro of the Hospital del Mar Medical Research Institute (IMIM), as a mentor and material provider, who has helped us with animal models etc. And we collaborate closely with experts in diagnostic imaging in Vall d’Hebrón and doctors from the Ramon y Cajal Institute
Here you can see a video (in English) where they tell us more about Nanotarg