The complexity of cells and tissues that form an animal body starts off as a deceitfully simple ball of embryonic stem cells (ESCs), the early embryo. How these cells lose their pluripotency (i.e. their ability to give rise to any type of cell), and differentiate into specific lineages is one of the mysteries of developmental biology. A mystery on which the research group led by Thomas Graf at the Centre for Genomic Regulation (CRG) has now shed some light.
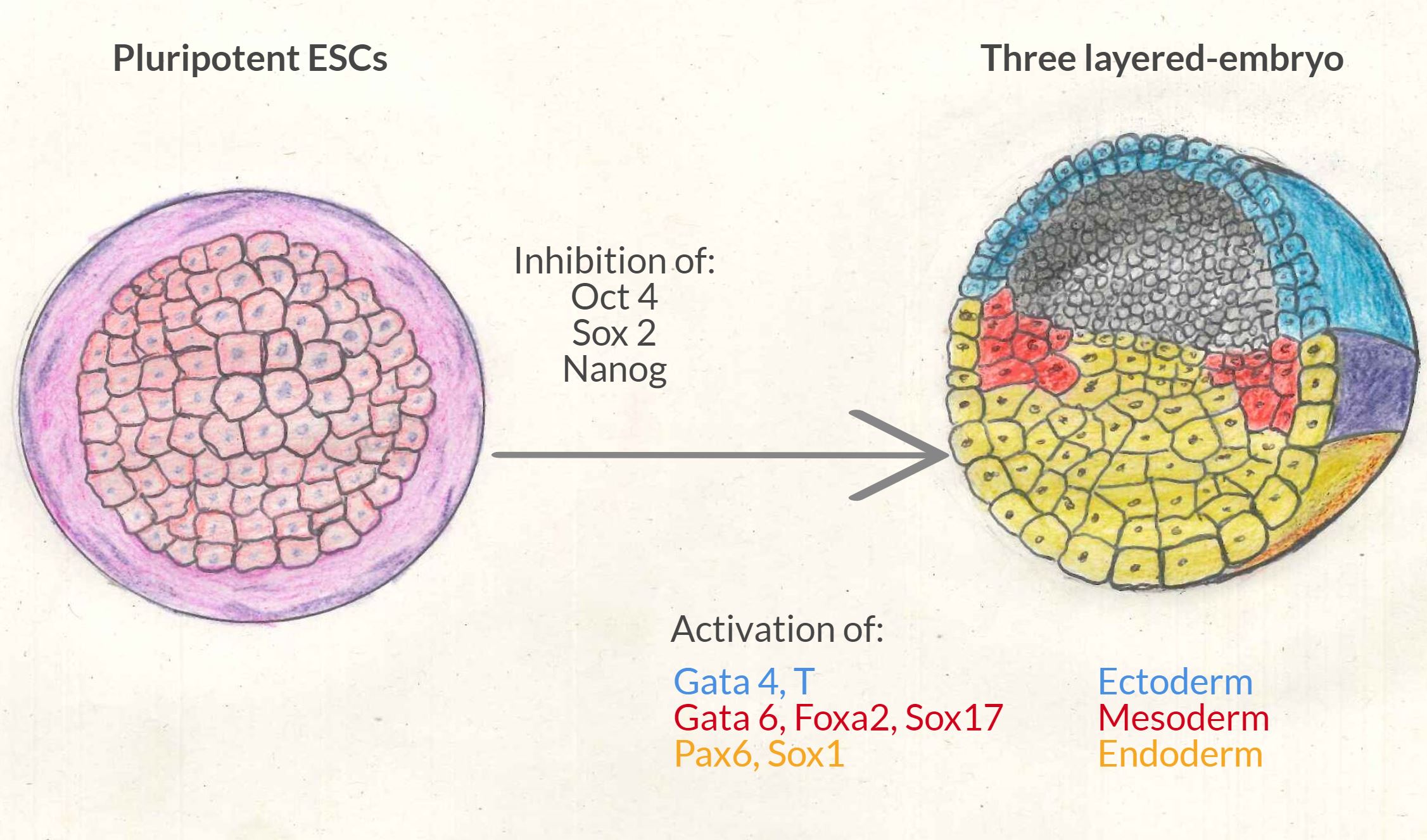
The vertebrate embryo starts as a uniform sphere of pluripotent cells (the embryonic stem cells, ESCs), which soon transforms, through a process called gastrulation, into a more complex structure with three layers of differentiated cell types. These three germ layers are called Endoderm (inner layer), Mesoderm (middle layer) and Ectoderm (outer layer), and they will give rise to all the cells and tissues of the adult organism.
- Endoderm (inner layer) → digestive and respiratory tracts
- Mesoderm (middle layer) → muscle, bones, dermis
- Ectoderm (outer layer) → epidermis, hair, nails, eyes and nervous system
The three germ layers of the embryo – Endoderm, Mesoderm and Ectoderm – will give rise to all the cells and tissues of the adult organism.
It was thought that the mechanisms by which embryonic stem cells (ESCs) lose their pluripotent status, and start differentiating into specific germ layers was a 2-step process:
- 1st pluripotency exit → ESCs lose their ability to become any type of cell. At the molecular level, this happens by the inhibition of the Oct 4, Sox 2, and nanog genes.
- 2nd lineage specification → ESCs differentiate into ectoderm, mesoderm or endoderm cells. At the molecular level this happens by activating a specific group of genes for each layer:
- Gata4 and Brachyury (T) in the case of mesoderm
- Gata6, Foxa2 and Sox17 in the case of endoderm
- Pax6 and Sox1 in the case of ectoderm
The inhibition and activation of these sets of genes makes the cell go from a relatively open chromatin (when the cell is pluripotent) to a more compacted one (when the cell is differentiated).
Now, Tian Tian and other colleagues at the Graf lab at the CRG, have found that pluripotency exit and germ layer specification might overlap, rather than happen one after the other, and they have discovered some critical players of the process. In particular, the scientists focused on the chromatin-related factor Whsc1, a histone methylase that has been associated with a rare form of genetic disease and the formation of multiple myeloma. The researchers found that:
- When Whsc1 is inhibited, mouse embryonic stem cells can not be efficiently differentiate into mesendoderm cells (although ectoderm is not affected), and pluripotency exit is delayed. This makes Whsc1 the first chromatin-related factor shown to be required for pluripotency exit.
- Whsc1 is an enzyme which can methylate histone. However, the function of Whsc1 in pluripotency exit and mesendoderm differentiation is independent of its catalytic domain.
- Whsc1 binds to regulatory elements of the lineage master regulators Gata4, T (Brachyury), Gata6 and Foxa2 and activates them, leading to germ cell specification. It does this together with Brd4.
The authors conclude that Whsc1 links silencing of the pluripotency regulatory network with activation of mesendoderm lineages, and is therefore a key player in this important transition in the embryo.
The Graf lab has found that chromatin-related factor Whsc1 is an important player in the differentiation of the early embryo; linking pluripotency exit with mesendoderm lineage specification.
“Our discovery overturns the old concept that pluripotent cells must first forget who they are before they can learn new rules that allow them to change their identity. Why and how Whsc1 only establishes a connection with two of the three germ layers is an interesting question for the future” says Thomas Graf, leader of this study, which was done in collaboration with Harvard University, the Whitehead Institute for Biomedical Research and the MIT, Cambridge, US.
Tian V. Tian et al. Whsc1 links pluripotency exit with mesendoderm specification. Nat Cell Biol. 2019 Jul;21(7):824-834. doi: 10.1038/s41556-019-0342-1. Epub 2019 Jun 24.