Some cellular dysfunctions and, consequently, some diseases, are due to the accumulation of misfolded proteins. However, cells have specific ways to try to solve this problem.
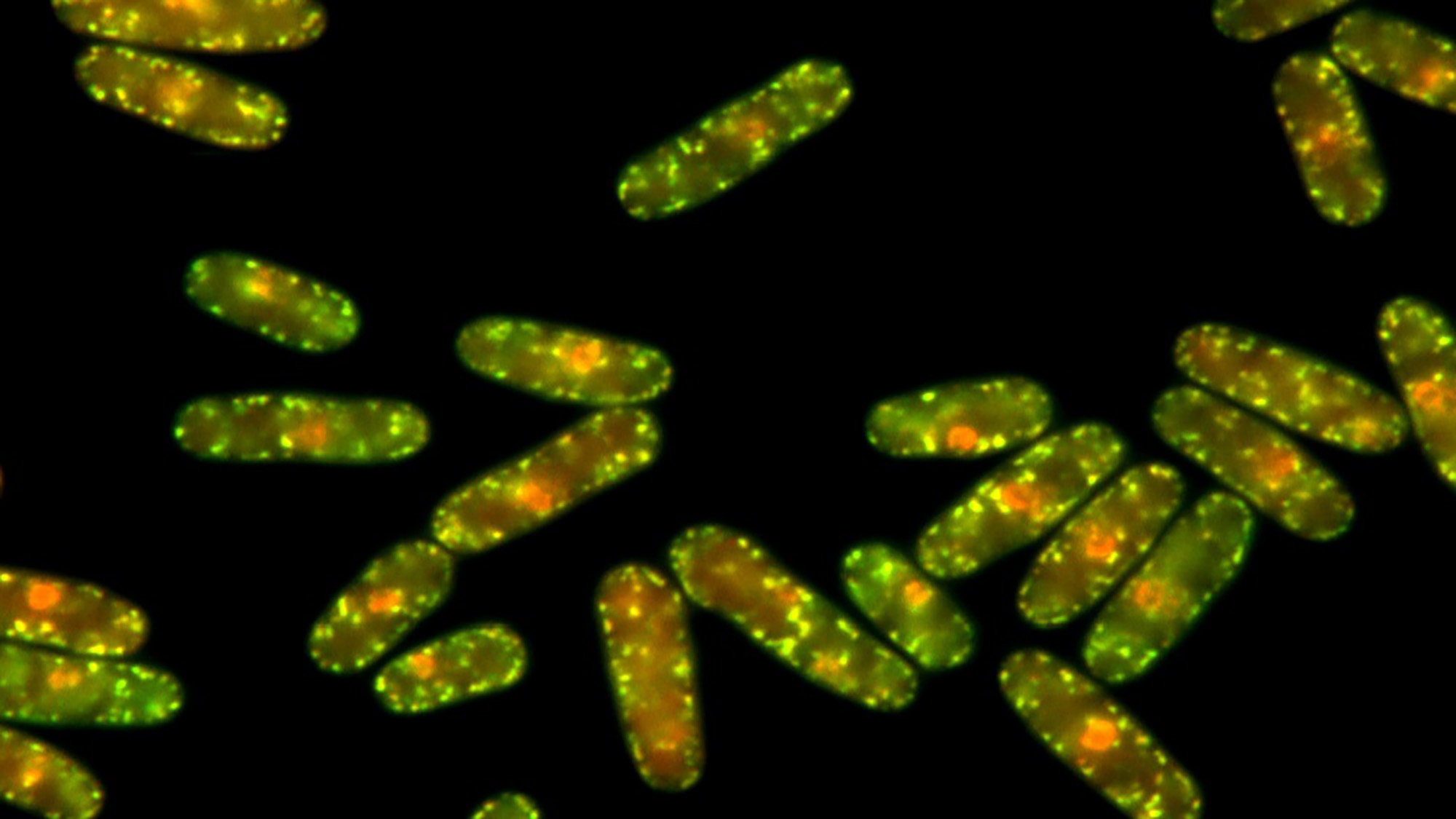
Researchers at the Department of Experimental and Health Sciences, Pompeu Fabra University (DCEXS-UPF) have recently confirmed in a study that the formation of protein aggregates would be the main strategy of cells to deal with this accumulation of misfolded proteins. This aggregation would prevent their degradation, so that once the conditions that caused the misfolding were overcome, the proteins could be properly folded.
Proteins must fold upon their formation to properly perform their functions. However, when cells are subjected to stress or harm, such as high temperatures, some of the proteins can misfold.
Chaperons are the small biological machines in charge of folding proteins. Nevertheless, under stress, they slightly change their role to promote aggregation. “Under stress conditions due to high temperatures, we have identified chaperones that play a key role in enhancing the aggregation route”, explains the co-first author of the study, Margarita Cabrera. “They detect that protein folding is not the solution and thus will actively promote the aggregation route”, she adds.
The laboratory led by Elena Hidalgo and José Ayté have used the Schizosaccharomyces pombe yeast model to investigate the protein quality control process, since this organism has the advantage of being simple, having easily manipulable genetics and basic molecular mechanisms applicable to eukaryotic organisms.
Cabrera, M., Boronat, S., Marte, L., Vega, M., Pérez, P., Ayté, J. and Hidalgo, E. February, 2020. Chaperone-facilitated aggregation of thermo-sensitive proteins shields them from degradation during heat stress. Cell Rep. doi: 10.1016/j.celrep.2020.01.077.