A study led by the Hospital del Mar Pharmacy Service in which the Neuropharmacology-Neurophar Research Group of the Department of Experimental and Health Sciences, Pompeu Fabra University (DCEXS-UPF) has collaborated, has verified that the mRNA of the SARS-CoV-2 vaccines is more stable than previously thought.
We have spoken with Elena Martín, from the Neurophar group, who tells us what the study consisted of and what consequences the results could have on the vaccination protocols.
Where did the project come from?
The idea came from the Hospital, since they were the ones who prepare the vaccines and they had to do it with great care so as not to alter the stability of the mRNA. Because they were so aware of the complications involved in the protocols, and knowing that there was no proof of the vaccines stability, they decided to check how stable the mRNA in Covid-19 vaccines actually was.
From there, Santi Grau, head of the Pharmacology Service of the Hospital del Mar and with whom we share the teaching of the pharmacology subject at UPF, raised the idea with Rafael Maldonado, director of the Neuropharmacology group. That’s when we decided to do an experiment to check the stability of the mRNA of the vaccines.
Going into more detail, what tests have you done?
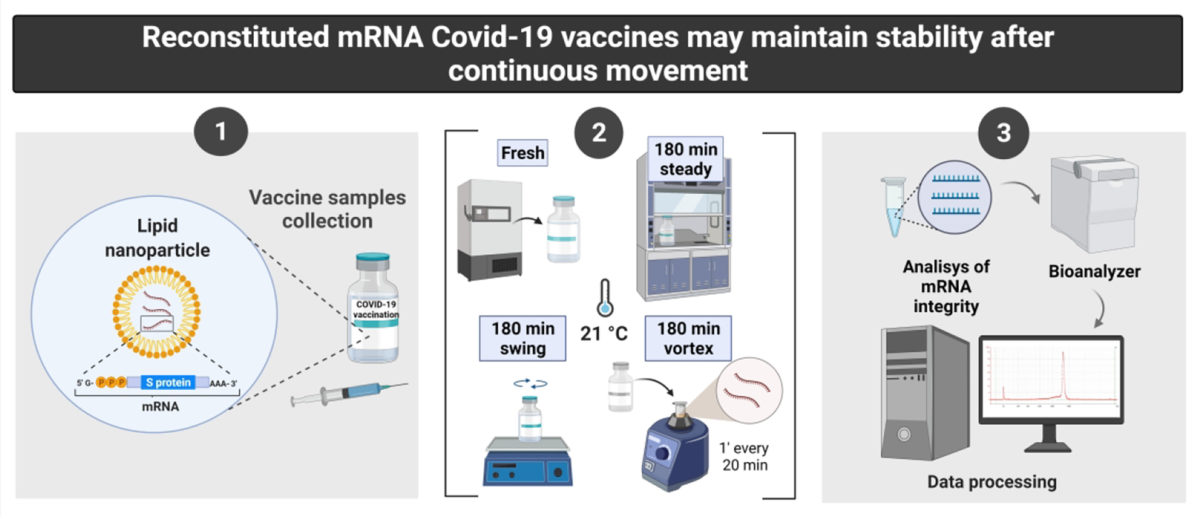
With the help of the UPF Genomics group we have carried out different tests in which we have analyzed and compared the stability of mRNA in 4 experimental groups:
- The control group was the sample that was thawed from frozen and immediately used, which we called the fresh sample, equivalent to the vaccines that are administered.
- A group that was in a shaker with a smooth movement, equivalent to a road transport.
- A third group agitated in a vortex, that is, subjected to much more intense stress.
- Finally, a fourth group of samples that were simply at room temperature, since checking how the “normal” temperature affected the stability of the mRNA was also important in order to evaluate the Hospital’s protocols.
When doing these tests on Moderna and Pfizer-BioNTech vaccines, we immediately verified that stability was preserved after 3 hours of gentle shaking, that is, that the group that was in a shaker with gentle movement at room temperature had a stability similar to that of that of the fresh group, the control group. The same was true for vaccines at room temperature, but not for those subjected to intense agitation.
With these results we have shown that, contrary to what was thought, the mRNA of the vaccines would remain stable in a transport by road of about 3 hours.
Can these results change vaccination protocols from now on?
Yes, especially in populations in remote areas or for people who, for different reasons, cannot access vaccination centers. We have verified that vaccines can be transported by road without problem, so that they can be prepared in reference centers and then administered in rural or difficult-to-access areas. Until now, people had to travel to the centers of reference, and now it may be the other way around, vaccines can be taken to them either by car, van or bike.
Knowing the mRNA is stable for 3h means that vaccines could be taken by road to people who cannot travel to the centers of reference
With this change in the protocol, we will be able to avoid that people in rural areas are not vaccinated due to the difficulty of the process (distance, travel time, etc.).
Knowing that the strict guidelines for the handling of vaccines have been one of the factors that has most determined the rate of vaccination of the population, how is it possible that this stability has not been verified before?
All the protocols that are applied in hospitals are derived from primary information offered by the companies responsible for vaccines, so out of precaution or ignorance they decided to take the measures that have been applied throughout this time.
Regarding our project, it is true that it could have been done before, but we must bear in mind that it takes a lot of time from the beginning of a study until it is published… In our case we had to send it to several journals to finally get it published, with all the revision process that implies.
But we are satisfied with the publication of the results: it was a nice surprise that the data was so clear and conclusive in terms of preserving mRNA stability!

Speaking of vaccines, I cannot avoid the question … What do you think of the use of information by the media on this whole issue?
It was all a bit chaotic. Every day news came out that pursued too much the novelty of the information rather than its veracity. Even so, I think it is positive that people are being made aware and, in general, a good informative work has been done.
The number of oogle searches on vaccines has now outnumbered those on coronavirus, which means that there is a lot of interest and that people are motivated to hear this type of information.
And to finish … You have mentioned the interest of the people, do you think that the population is more prepared and attentive to scientific information after all we have gone through?
I think so. I think that the effort of the scientific community is now much more valued and with the Covid-19 crisis it has been seen that promoting research can go a long way, it has set a precedent. Now people are aware that by investing time, money, infrastructure and personnel in other diseases, very important advances could be achieved.
Now people are aware that by investing time, money, infrastructure and personnel in other diseases, very important advances could be achieved.
Now the whole population knows terms like mRNA or PCR, so in general, I think science and the importance of scientific research has been revalued.
Grau S, Ferrández O, Martín-García E, Maldonado R. Reconstituted mRNA Covid-19 vaccines may maintain stability after continuous movement, Clinical Microbiology and Infection, 2021