The development of a new drug with the characteristics of efficacy and safety required today is a long and costly process. Typically, the process can last for more than 12 years and require highly risky investments superior to one billion euros.
In this scenario, it is not surprising that the pharmaceutical companies are open to incorporate novel technological advances, with the hope of making the process faster and more efficient. The term “computer-assisted drug design” (CADD) encompasses a wide range of technologies which have in common the application of computational methods at diverse points of the drug development pipeline.
The process of drug development can last for more than 12 years and requires highly risky investments of more than one billion euros.
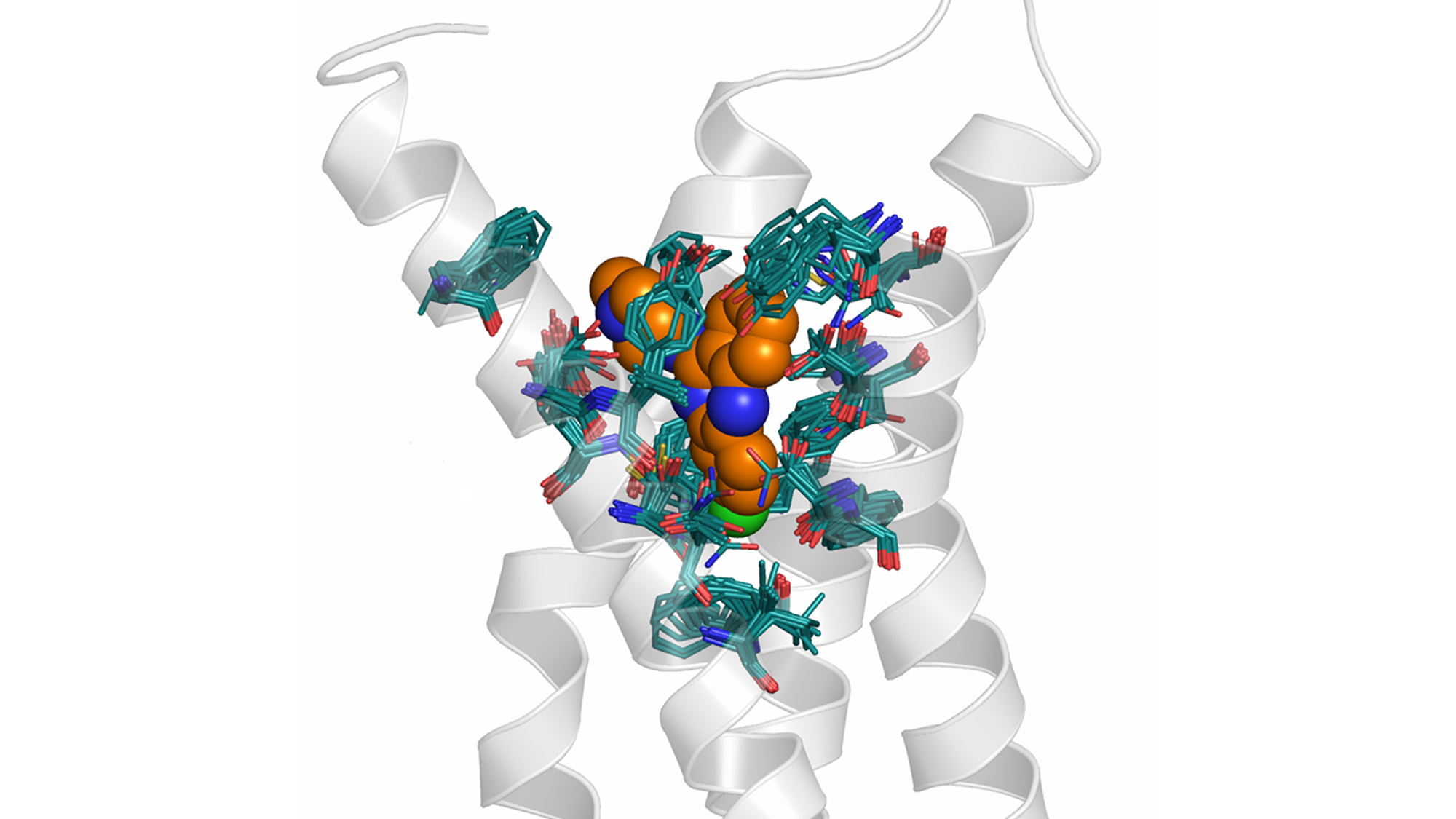
Focusing on the preclinical stages, CADD techniques are typically applied in the search of drug candidates with a certain profile of biological properties. When the structure of a biological receptor is known, direct methodologies can exploit this information to search for molecules that fit well the receptor binding site. This can be done by different methods, for example assembling fragments which complement the shape and physico-chemical properties of the receptor, or searching for a suitable compound in databases storing millions of structures. When the structure of the receptor is not available, but a few low-active compounds are known, it is still possible to apply methods based on molecular similarity to obtain better compounds.
In practice, the methods are more complex because several biological properties must be considered and optimized simultaneously. Indeed, modern approaches to drug design do not consider a single target but the interaction of the candidate with many receptors, some associated with desirable properties and others associated with undesirable side effects.
Article written by Manuel Pastor, researcher at the Department of Experimental and Health Sciences, Pompeu Fabra University (DCEXS-UPF).