It is common knowledge that bacteria can develop antibiotic resistance mechanisms. It is a natural phenomenon that, due to the misuse of these medicines, is being accelerated.
Bacterial resistance mechanisms reverse the process by which the antibiotic alters their structure or functionality. Depending on the mechanisms developed by each bacteria, they will show a higher or lower resistance to some antibiotics. However, can these microorganisms change their tolerance to antibiotics depending on whether or not they live with another bacterial species? A new study led by Leticia Galera-Laporta and Jordi Garcia-Ojalvo, researchers at the Department of Experimental and Health Sciences, Pompeu Fabra University (DCEXS-UPF), confirms it.
In this study, the researchers examined how communities of multiple species of bacteria — such as found in infections and microbiota, for example — respond jointly to antibiotics.
It is important to bear in mind the fact that bacteria’s survival of antibiotics can be due to non-genetic mechanisms.
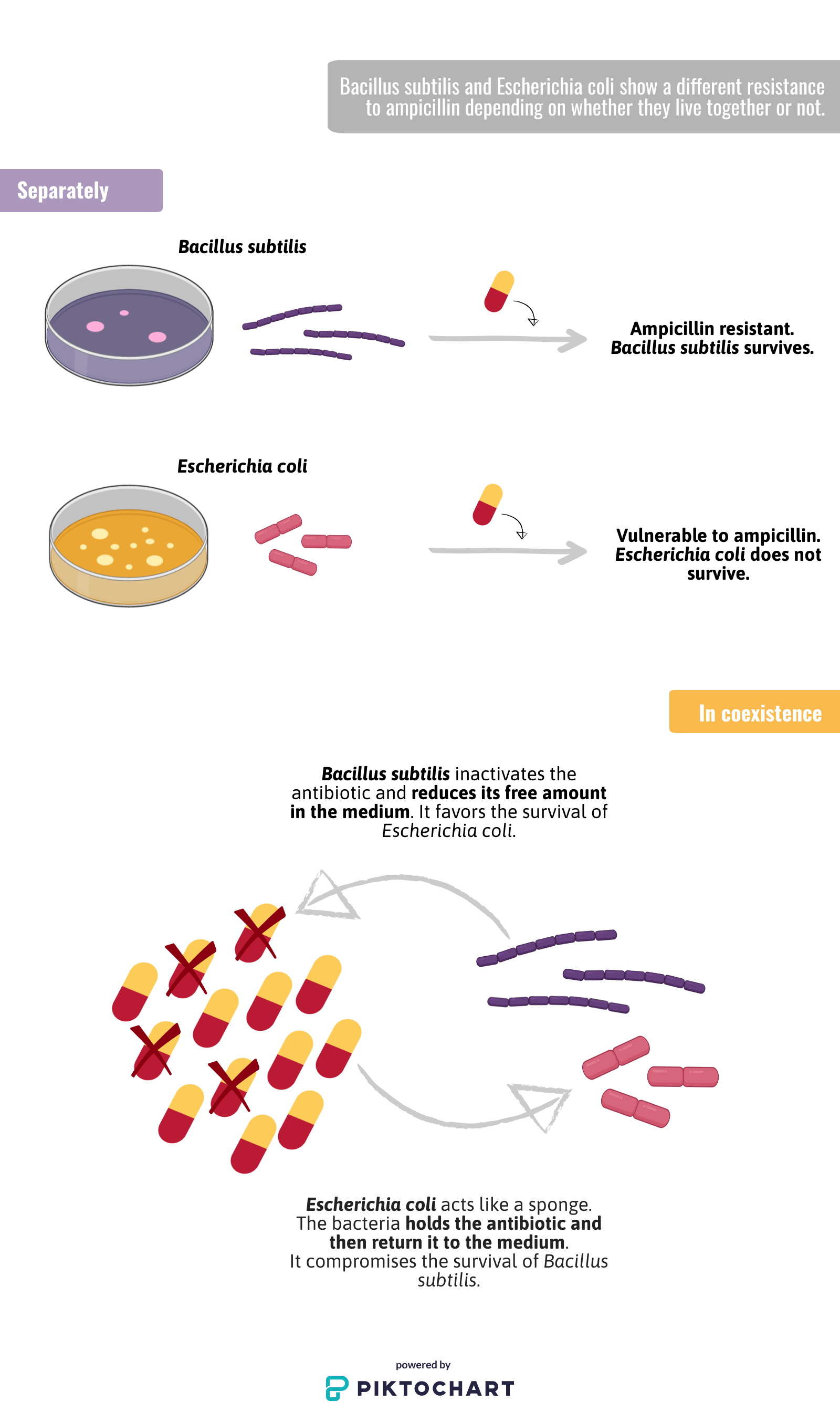
To answer this question, they studied how Bacillus subtilis and Escherichia coli respond to ampicillin (penicillin-type antibiotic); on their own, E. coli is sensitive to the antibiotic, while B. subtilis is resistant. Nevertheless, when both bacterial species coexist, their response to ampicillin is reversed. Thanks to a mathematical model the researchers could see that what really changes is the collective response; when both bacteria coexist, the availability of the medicine in the environment changes and, consequently, so does the tolerance of E. coli and B. subtilis to the antibiotic.
“We must consider the microbial context in which the bacteria are found, in order to improve the information that enables choosing the appropriate dose of antibiotic in each case”, concludes Garcia-Ojalvo.
L. Galera-Laporta i J. Garcia-Ojalvo, “Antithetic population response to antibiotics in a polybacterial community”. Science Advances, March 2020. https://doi.org/10.1126/sciadv.aaz5108