Malaria remains a significant global health and economic challenge, disproportionately affecting African children in its most severe forms. Now, a new study in collaboration with the Bernabeu group at the European Molecular Biology Laboratory – Barcelona (EMBL Barcelona) has identified human antibodies capable of recognizing and targeting key proteins implicated in severe malaria.
The parasite that causes the disease, Plasmodium falciparum, infects the blood cells and modifies them to adhere to the walls of brain microvessels. This adhesion impairs blood flow, blocks tiny vessels, and can lead to brain swelling and the development of cerebral malaria.
Gradually, children develop immunity to malaria as they age, with adults rarely experiencing the lethal effects of the disease. It is expected that this protection is related to antibodies that target virulent proteins present on the surface of infected red blood cells. There are about 60 proteins from that family, called PfEMP1. However, the diversity of PfEMP1 variants has made it a challenging target for vaccine development.
Mimicking human blood vessels in the lab
Researchers wondered whether the human immune system could generate antibodies that target all variants of PfEMP1 in circulation. A team from the University of Texas identified two promising antibodies that bind to a specific region of PfEMP1, which interacts with EPCR receptors, critical proteins on red blood cell surfaces where the parasite’s virulent proteins attach.
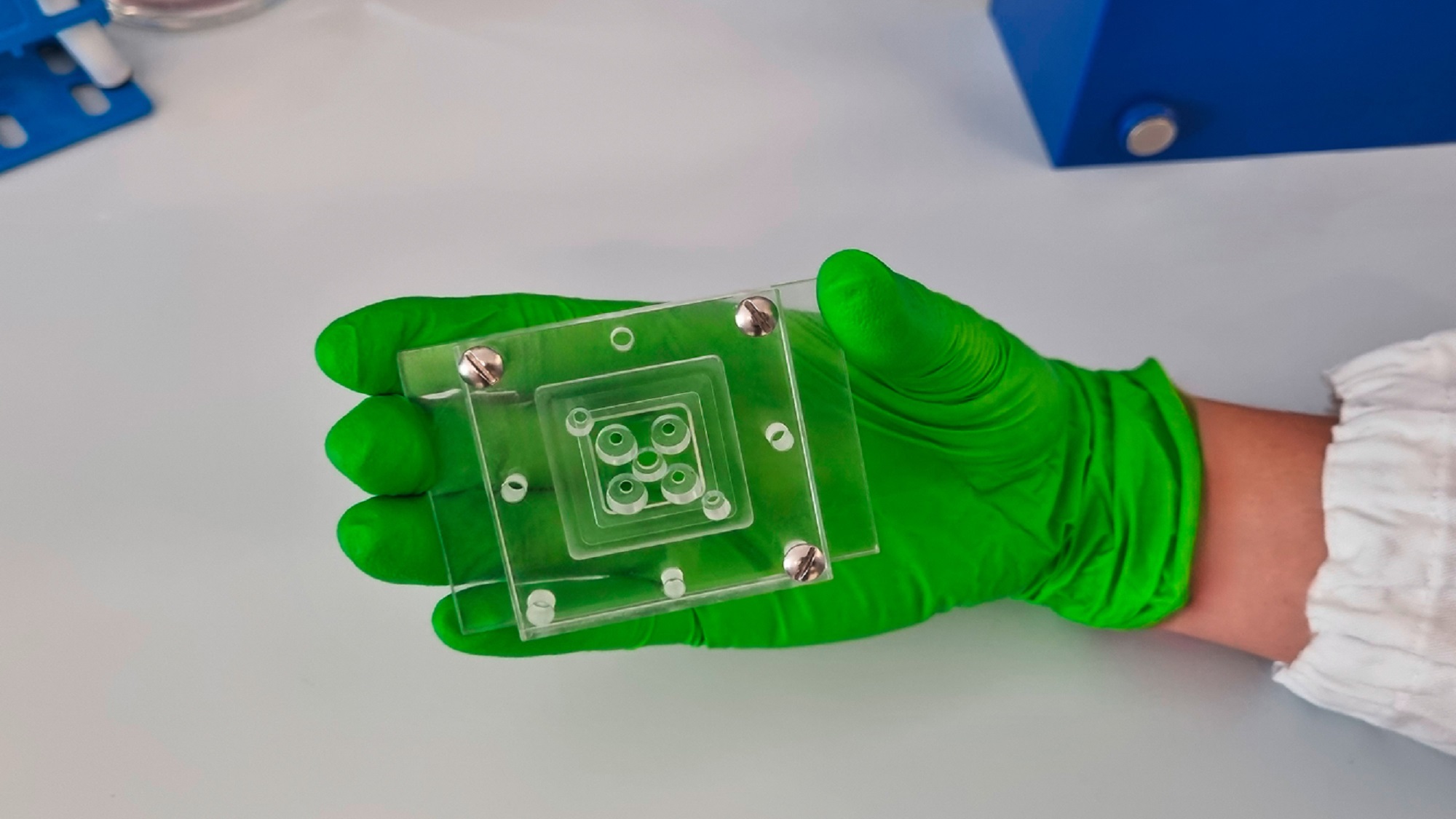
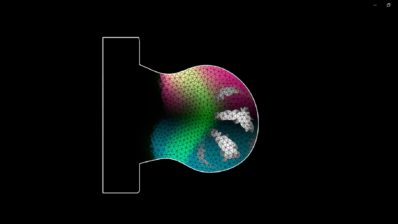
Testing these antibodies in traditional model organisms was impossible, as the parasite only infects humans, and model animals do not express PfEMP1. So far, efficacy studies can only be done in vitro. That is why researchers used organ-on-a-chip technology. They created a network of human blood vessels in 3D in a dish to perfuse them with malaria-infected blood, and see the antibodies’ action. Both antibodies were able to prevent the accumulation of infected cells, suggesting they might help stop the blockage that leads to severe malaria symptoms.
“We introduced the two antibodies into the vasculature and were impressed at how well they prevented infected blood cells from sticking to the vessels. It was striking to see inhibition readily apparent by eye”
Viola Introini, co-first author of the work (EMBL Barcelona)
Asking Bernabeu if these antibodies could be useful against malaria in general, she says they are mostly aiming to prevent severe disease. Right now, there are 2 malaria vaccines available and recommended by the WHO that target the malaria parasite in the liver, before it reaches the blood and causes severe symptoms. Nevertheless, they are not fully effective. There are other vaccine targets in clinical trials that are quite promising. However, because the malaria parasite is so complex and variable, it could be possible that at the end we will need a combination strategy that targets and weakens multiple points on the malaria life cycle.
A complex path ahead
The researchers acknowledge that translating these findings to real-world treatments requires further steps. “What we see in these organ-on-chip microvessels provides a good starting point, but there’s a lot more work to do. In this study, we tested basal conditions. Future work will include simulating inflammation and fever, as these are critical during severe malaria,” explains Bernabeu.
For now, efficacy studies rely on in vitro models, but the team plans to expand their screening efforts to identify more antibodies capable of targeting other classes of PfEMP1 that cause severe disease. It even can be possible that at some point, they can feed all this information to AI models to design the most efficient antibodies.
The study was highly collaborative with the contributions from different groups being key for the success of the project. From immunologists at the University of Texas that developed new antibody purification tools from donors, and structural biologists in San Diego (the Scripps Research Institute), Copenhagen (University of Copenhagen) and Seattle (the Fred Hutchinson Cancer Center) that developed cryo-EM studies and protein-protein binding studies – to the team led by Bernabeu at the PRBB, where they did and tested the microvessel chips.
And all the participating teams are mindful of the broader implications of their work. With malaria’s burden concentrated in low-resource settings, affordability and accessibility remain paramount. “If our studies continue to succeed, it will be essential to ensure that any resulting treatments or preventive strategies are affordable to everyone,” concludes the head of the group.
Reyes, R.A., Raghavan, S.S.R., Hurlburt, N.K. et al. Broadly inhibitory antibodies to severe malaria virulence proteins. Nature 636, 182–189 (2024). https://doi: 10.1038/s41586-024-08220-3