Susanne van den Brink is one of the pioneers of biomedical research using embryo models. She currently works in the Stem Cells and Cancer laboratory led by Anna Bigas at the Hospital del Mar Research Institute.
Embryo models – or embryonic organoids – are three-dimensional cultures of stem cells that resemble stages of embryonic development. Most of the currently available embryo models reproduce only part of the embryo. They allow us to better understand embryonic development, and in the future, it might be possible to use them to create tissues and cells for transplantation purposes.
In this interview, van den Brink explains how she is developing an embryo model that could be used to produce blood stem cells. Those could replace donor-derived blood stem cells in bone marrow transplants, which are used in the clinic to treat diseases such as leukaemia. We also talk to her about what it means to be a pioneer and about scientific ethics.
How did your work with embryo models begin?
During my Master internship in Alfonso Martinez Arias‘s lab at the University of Cambridge, we developed the first embryonic mouse model, which was partly a serendipity. We combined two stem cell culture protocols and accidentally produced three-dimensional round structures that elongated.
We were at first confused about these structures and decided to film them under the microscope for 24 hours. The next day we confirmed my supervisor’s suspicion that it was an embryo-like structure. We were very excited about this and called people from other labs to come and see our results. It was a very surreal internship; I was very lucky.
“During my Master internship in Alfonso Martinez Arias’s lab at the University of Cambridge, we developed the first embryonic mouse model, which was partly a serendipity”
Susanne van den Brink (Hospital del Mar Research Institute)
We published the embryo model in 2014 and I continued to develop these models during my PhD at the Hubrecht Institute in the Netherlands. During my PhD, we collaborated with Alfonso Martinez Arias in Cambridge and together we developed the first human version of this model.
I decided to come to the PRBB for my postdoc on Alfonso’s recommendation – who had recently also moved here – and because I was really impressed by the imaging facilities here. There are very good microscopes in the park, which are shared by all the research centres working here.
Could you tell us more about what embryo models are?
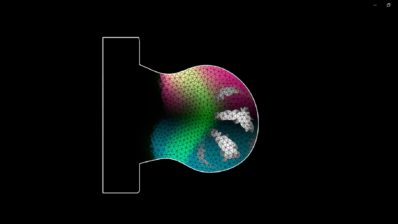
Embryo models are three-dimensional structures that resemble certain stages of mouse or human embryonic development. We create them in the lab without fertilisation but using stem cells. The types of embryo models that we are working with are incomplete models because they cannot produce some vital parts of the embryo, such as the extraembryonic tissues or some parts of the body. They are therefore not viable because they cannot develop into an individual.
They are only used for a few days to study a specific stage of embryonic development. Laboratories around the world are developing different embryo models that resemble different developmental stages or different parts of the embryo.
What type of embryo model do you use?
We work with mouse gastruloids, which mimic part of the gastrulation process. During gastrulation, the embryo establishes the body axis. It elongates and starts to define what the head, tail, left, right, dorsal and ventral parts of the body will be. This stage takes place between day 6.5 and 8.5 after fertilisation in the mouse and starts on day 16 in humans.
We keep the human gastruloids in culture for up four days; mouse gastruloids can be kept in culture for up to nine days. The gastruloids do not have the part of the embryo that develops anterior structures such as the brain, nor the extraembryonic tissues that form structures such as the placenta. They are therefore not viable, as they can never be implanted in the uterus or develop into a full individual. This is very important from an ethical point of view.
The ethical issue is certainly very important in this kind of research, and we will come back to that later. But first, can you tell us about the applications of these embryo models?
They can be used to study embryonic development, to test the toxicity of drugs and foods during pregnancy on embryonic development, and in the future perhaps also to develop tissues for transplantation purposes. When scientists study embryonic development, they usually use animal models such as mice. They have been extremely useful, but they have some limitations. For example, mouse embryos cannot be obtained in large numbers, which makes it difficult to perform large-scale screenings (toxicity and drugs testing), and results obtained in mice are not always relevant to humans.
“When scientists study embryonic development, they usually use animal models such as mice. They have been extremely useful, but they have some limitations.”
This was the case in the 1950s with thalidomide, a drug used to reduce morning sickness in pregnant women. It was tested on mice, so they thought it was safe. But then they found out that it’s actually very dangerous in humans, and it resulted in newborns with severe deformities. Using human embryo models could have prevented this.
Embryo models can also be used to study stages of human embryonic development that cannot be studied with natural human embryos. Today, in most countries, we can only study viable human embryos (‘leftovers’ from assisted reproduction) up to day 14, or use abortion material, which is usually available from week 4. Therefore, our knowledge about human development between day 14 and week 4 is currently very limited. Human embryo models can be used to study the essential processes, such as gastrulation, that happen in this period.
What is your research with embryo models about? What are you trying to do?
We are working with the gastruloid model and trying to generate blood stem cells. These could be used in bone marrow transplants to treat blood diseases such as leukaemia. This would allow us to avoid several of the problems associated with donor-derived bone marrow transplants, such as the difficulty of finding immune-matched donors.
We are trying to model the AGM (aorta-gonad-mesonephros) region of the mouse embryo, where the blood stem cells are produced during embryogenesis. If we are successful with the mouse, we will try it with human cells.
If it works, we could make personalised blood stem cells from a patient’s skin cell. But since that would also be expensive, we’re also thinking about alternative methods. Perhaps we could make 40 lines of embryo models with the 40 most common blood types; or focus on the types for which finding matching donors is challenging. We are collaborating with the Catalan and Dutch blood banks because they know the needs better.
It is already possible to generate tissues using organoid cultures, models that mimic organs. Why is it more interesting to generate these tissues using embryonic models?
Yes, that’s a very good question. An organoid only reproduces a specific organ. An embryo model, or embryonic organoid, reproduces a whole part of the embryo, including the organ of interest and all the surrounding tissues. This is important because tissues interact with each other. For example, in our case, blood stem cells develop in the embryonic aorta, and tissues close to the aorta secrete signals that influence the development of blood stem cells. Therefore, we will probably not only need the aorta, but also the gonads and the mesonephros (embryonic kidneys), which develop close to the aorta, to generate transplantable blood stem cells.
There are very strict ethical considerations and laws regarding human embryo research. How do they apply to the embryo models?
I’m very interested in ethics. Most scientists I’ve seen are very careful themselves: we want to do things that improve society, and we don’t want to damage the public image of our field. Furthermore, before we do any new experiment involving humans or animals, we must go through ethics committees.
“We want to do things that improve society, and we don’t want to damage the public image of our field. Furthermore, before we do any new experiment involving humans or animals, we must go through ethics committes.”
In most countries, the law does not allow human embryos created by in vitro fertilisation (IVF) to be kept alive beyond the 14th day after fertilisation. This is just before gastrulation. For embryo models, there are still no laws in most countries, but the scientific community generally accepts the ethical guidelines of the International Society for Stem Cell Research (ISSCR). These say, among other things, that human embryo models should never be transferred into the uterus of a human or animal.
I am working with the Dutch government, which is starting to draft the first version of their human embryo model law. Drafting such a law is very complicated. The government is organising debates with the society. But if you want an informed opinion from the general public, you first have to spend time explaining the concepts, and that sometimes proves to be very difficult.
So you are working with mouse models, but you don’t know if it will be legal to move on to human models…
That’s right. Although it would probably be allowed as we only want to model the AGM region, not the whole embryo. The ethics committee thinks it is ethical to make human gastruloids even though they model post 14-days old human embryos, because they lack extra embryonic tissues and anterior neural structures, so they cannot implant or generate the brain.
In any case, I am also personally very interested in how the mouse embryo builds up the AGM region. By trying to make this mouse model, we are learning a lot about embryonic development and building fundamental knowledge.
What are your biggest research challenges?
We want to build something that no one has generated before, and therefore, we have no idea how to do it. The only possible strategy is to try as many things as possible. Sometimes we base our experiments on literature knowledge about embryogenesis, but it’s also a lot of just trying things.
“We want to build something that no one has generated before, and therefore, we have no idea how to do it. And because we are pioneers, we cannot always predict what will happen”
We also have problems with reproducibility, and that’s very important. Sometimes I manage to create something that looks really beautiful, and then the next day I do exactly the same thing, but I cannot reproduce it. Pioneering is a lot of fun, but it’s also difficult. It takes a lot of patience. And it’s slow.
Finally, there is a side note on the ethical issues. We ask permission from the ethics committees, but because we are pioneers, we cannot always predict what will happen. I made this gastruloid model for the first time during my internship by accident. That’s a complication in the general field, sometimes you don’t know what you’re going to do until you’ve done it.
You talk very passionately about your work!
Yes, I thought a lot about changing fields, and I actually did for a while. But I really missed this field. I really like these models. I just find it so fascinating that cells can spontaneously self-organise into a structure that makes up parts of the embryo.
We have movies where you can see our gastruloid embryo models forming and elongating under the microscope, and it’s so amazing. The cells can do that on their own and we can see how they interact with each other to form complex structures. And our embryo models help us to better understand these fascinating and complex processes that drive early mouse and human embryonic development.