Two recent recent studies from the Centre for Genomic Regulation (CRG) have pointed to a gene that could be a potential new target to block metastatis in melanoma – and to over 50 involved in mantle cell lymphoma.
Preventing metastatic melanoma
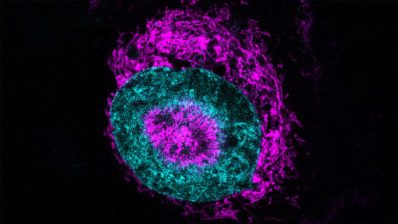
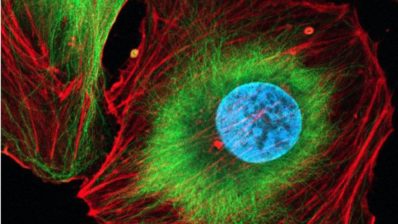
The first study, led by Fátima Gebauer, has identified the protein eIF2A as a critical driver of melanoma cell migration, offering a potential therapeutic target to prevent metastasis. While traditionally linked to protein synthesis under stress, eIF2A was found instead to guide centrosome orientation, enabling malignant cells to move through tissues and spread.
“We found that eIF2A works to preserve parts of the centrosome so it points the cell in the right direction during movement”
Jennifer Jungfleisch (CRG) first author of the study.
Disabling eIF2A in metastatic melanoma cell lines halted tumour sphere growth and impaired migration without majorly affecting protein production. Crucially, eIF2A dependence appears only after malignant transformation – that is, is is indispensable only when cells become metastatic – suggesting a therapeutic window where healthy tissues may be spared. Researchers highlight that targeting the protein’s tail, essential for centrosome function, could block the melanoma’s spread and open new strategies to combat melanoma metastasis.
Chromosomal translocation in mantle cell lymphoma
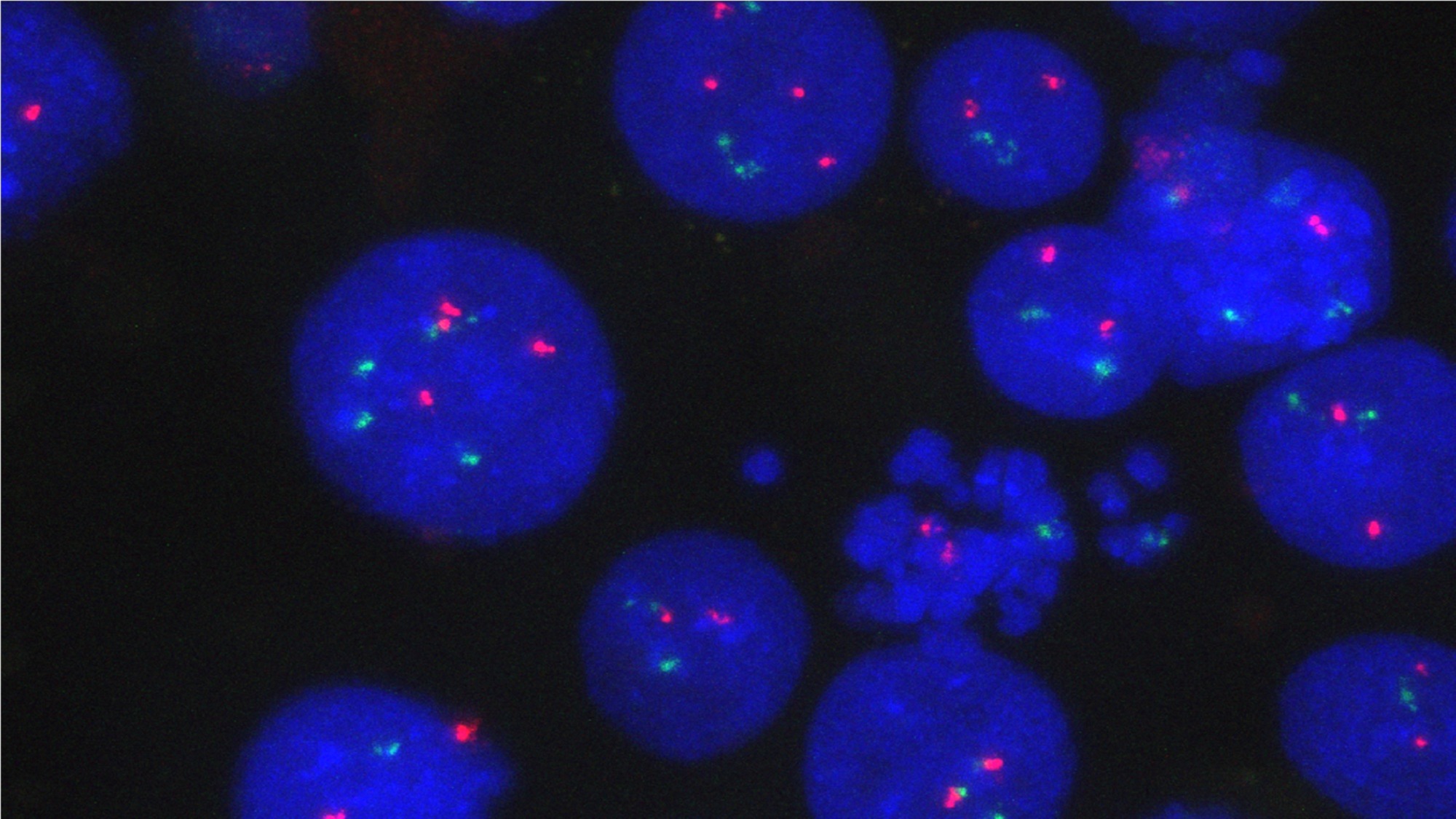
A second study from the CRG, this one led by Renée Beekman reveals a new way chromosomal translocation can promote cancer. Her team used CRISPR to recreate the chromosome break most seen in patients with mantle cell lymphoma, a rare but aggressive subtype of lymphoma, where a piece of chromosome 14 swaps places with a piece of chromosome 11. It was known that this translocation brings the gene regulatory element IGH enhancer, which normally boosts the activity of antibody production in healthy B cells, right beside CCND1, a gene which helps cells divide. The enhancer treats CCND1 as if it were a gene encoding for antibodies, increasing its expression and leading to cancer.
But increased CCND1 activity alone does not start the lymphoma. Instead, the researchers found that in its new position, the IGH enhancer boosts the activity of 50 genes at once.
“The translocation relocates the IGH enhancer into a DNA loop that amplifies gene expression over 50 million base pairs, effectively rewiring the genome”
Anna Oncins (CRG and MELIS-UPF), first author of the study.
This mechanism greatly expands the number of potential therapeutic targets, and the authors now plan to study exactly how the newly identified genes contribute to the initiation and progression of lymphoma.
Jungfleisch J, Mestre-Farràs N, Gómez-Riera R, Pourcelot O, Bertrand E, Halidi N, Gebauer F. eIF2A regulates cell migration in a translation-independent manner. Sci Adv. 2025 Aug;11(31):eadu5668. doi: 10.1126/sciadv.adu5668. Epub 2025 Aug 1. PMID: 40749049; PMCID: PMC12315956.
Oncins A, Zaurin R, Toukabri H, Quililan K, Hernández Mora JR, Karpinska MA, Wernersson E, Smith A, Bianchi A, Albinati L, Cozzuto L, Rivero A, Gulliver C, Velten J, Denkena J, Serra F, Gómez R, López C, Beà S, Paulsen J, Halidi N, Valencia A, Bienko M, Oudelaar AM, Beekman R. Translocations can drive expression changes of multiple genes in regulons covering entire chromosome arms. Nucleic Acids Res. 2025 Aug 11;53(15):gkaf677. doi: 10.1093/nar/gkaf677. PMID: 40840919; PMCID: PMC12370299.