Antibiotic resistance is a pressing global health issue, and researchers from Jordi Garcia-Ojalvo‘s lab at the Department of Medicine and Life Sciences (MELIS-UPF), in collaboration with scientists the University of California San Diego, have uncovered a novel strategy to address it.
The study reveals that limiting magnesium (Mg²⁺) availability to antibiotic-resistant bacteria could hinder their survival and prevent the spread of resistance without the need to develop new antibiotics.
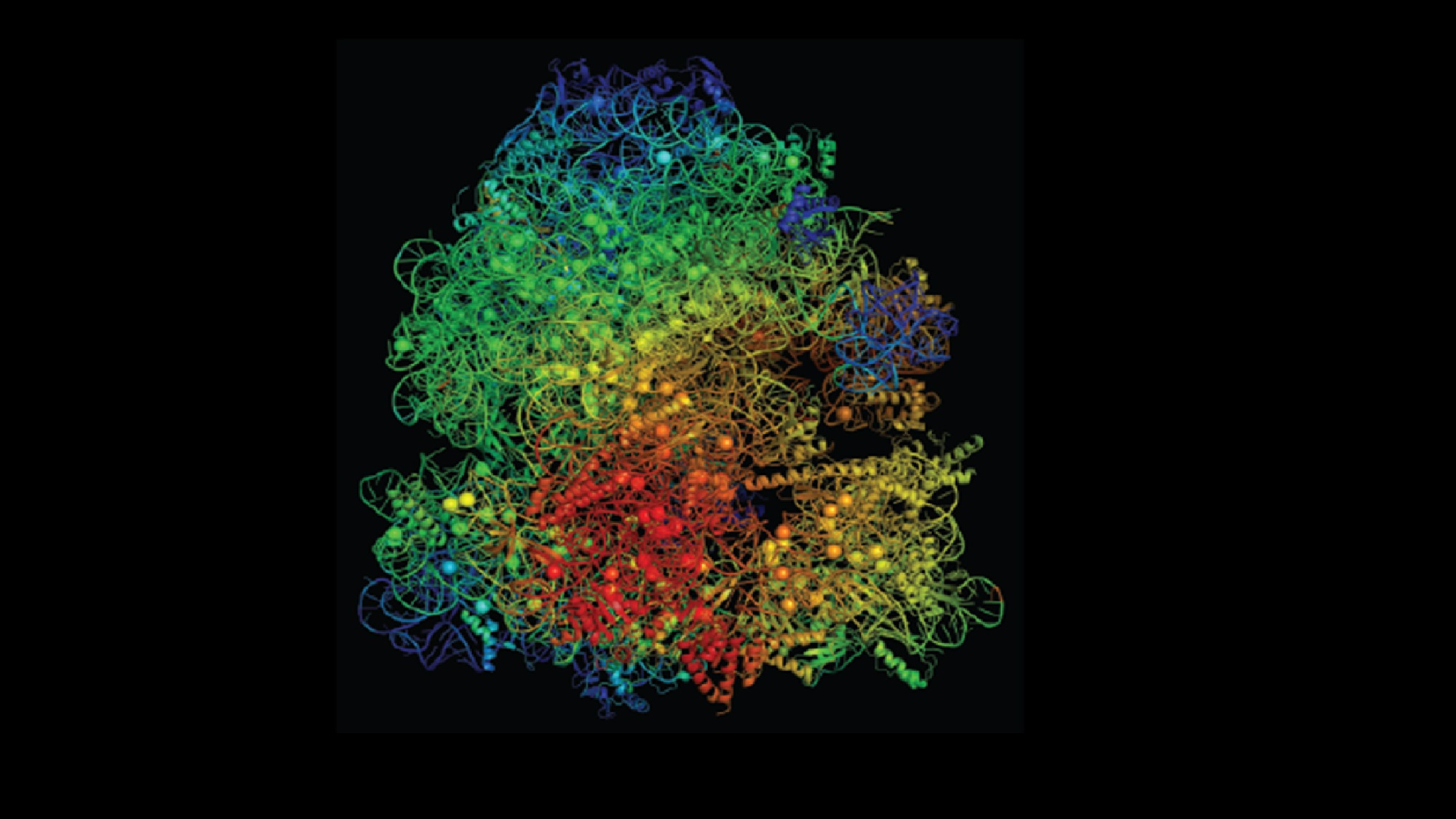
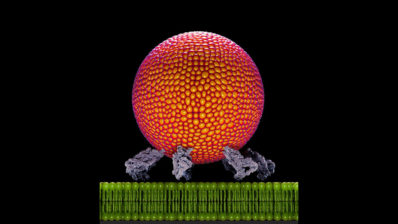
The research focuses on the ribosome, a critical cell component targeted by antibiotics like erythromycin. A mutant strain of Bacillus subtilis (L22) has ribosomal alterations that confer antibiotic resistance. However, these mutations impose a physiological cost: the mutant ribosomes accumulate more magnesium, leaving less available for essential processes such as ATP production, which is crucial for cellular energy. This disadvantage becomes pronounced when environmental magnesium is scarce, restricting the mutant bacteria’s survival compared to non-mutated strains.
Limiting magnesium levels in the environment could help limit the spread of antibiotic-resistant strains.
Using computational models, researchers confirmed the connection between Mg²⁺ dynamics and bacterial fitness. The findings suggest that manipulating magnesium levels in the environment could serve as an innovative approach to limit the spread of antibiotic-resistant strains, offering a new dimension to the fight against antimicrobial resistance.
Chae Moon E, Modi T, D Lee D-Y, Yangaliev D, Garcia-Ojalvo J, Ozkan SB, Gürol MS. Physiological cost of antibiotic resistance: Insights from a ribosome variant in bacteria. https://www.science.org/doi/10.1126/sciadv.adq5249