This text was written by Júlia Urgel, a PhD student led by Ana Janic at the Department of Medicine and Life Sciences (MELIS-UPF) and Sara Sdelci at the Centre for Genomic Regulation (CRG).
Júlia won the audience award in the Rin4′ science outreach contest with her presentation “Lung cancer, what could you not live without?”. Here, she tells us more about her research and what led her to it.
When I was only a teenager, I read that half of the people in the world will suffer from cancer at least once in their lives and one-third of the patients will die because of it. This shocking realization prompted me to contribute to the fight against cancer.
A year after I made this decision, my family entered cancer’s statistics. Seeing numbers turning into names, faces and feelings convinced me to pursue a career in cancer research.
“When my family entered cancer’s statistics, I was convinced to pursue a career in cancer research”
Júlia Urgel (MELIS-UPF)
Since then, I have been paving my way until reaching the point of developing a PhD project in the deathliest cancer type and the first case of cancer that occurred in my direct environment: lung cancer.
Lung cancer is the leading cause of cancer death globally, counting for 1,8 M deaths in 2022. TP53 and KRAS are the two most mutated genes in lung cancer and their co-occurring mutation leads to a more aggressive phenotype with potentially poorer prognosis. TP53 is the most important tumour suppressor, also known as the guardian of the genome, which means that it regulates cell division by keeping cells from growing and dividing. On the other hand, KRAS is an oncogene, which means that when there is a mutation in KRAS, it signals too much and cells grow without being told to, which causes cancer.
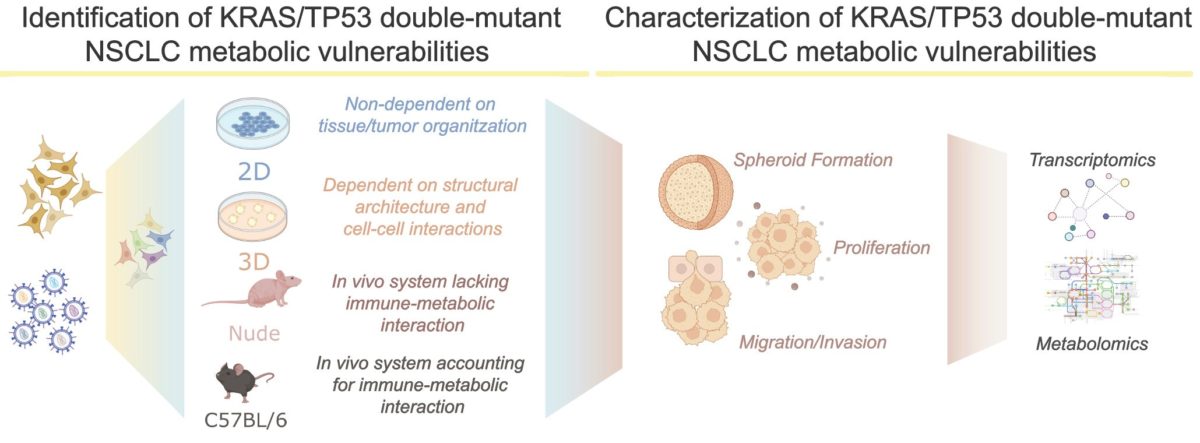
When studied separately, TP53 or KRAS dysfunctional lung cancers are known to undergo a deep metabolic adaptation, differently from the normal (non-mutated) version of the genes. However, although they constitute nearly 15% of the non-small cell lung cancer cases, very little is described about the metabolic changes (also known as metabolic rewiring) occurring in tumours harbouring mutations in both genes. Given the fact that metabolic rewiring is one of the hallmarks of cancer, we hypothesize that KRAS/TP53 double-mutant tumours change cellular metabolism to sustain their proliferation. Thus, my prospective PhD project focuses on the identification and characterization of the essential metabolic genes involved in the survival of lung tumours driven by the mutation of these two genes. Summarizing:
- TP53 mutant –> known deep metabolic rewiring
- KRAS mutant –> known deep metabolic rewiring
- TP53 + KRAS mutants –> unknown deep metabolic rewiring
If our hypothesis is correct, certain metabolic factors may be specifically essential in KRAS/TP53 double-mutant tumours, thus behaving as metabolic vulnerabilities: genes of the metabolism whose loss promotes the death of the cancer cells with these mutations. By using human and mouse Non-Small Cell Lung Cancer (NSCLC) models, we will set up a series of experiments to dissect the metabolic rewiring that supports the proliferation of KRAS/TP53 double-mutant tumours, focusing on metabolism-centred genetic screenings.
The information provided by this study might shed some light into the molecular understanding of metabolic pathways in this specific type of lung cancer, maybe leading to further therapeutic strategies. Moreover, our results might be potentially validated in other types of tumours where the coexistence of TP53 and KRAS mutations has been observed (for example pancreatic tumours).
You can listen to Júlia’s 4-minute talk in this video (in Spanish)