Cerebral malaria – when the Plasmodium falciparum infection manages to traverse the brain-blood barrier (BBB) and get to the brain – is a severe complication of this tropical disease, and it has high mortality rates, even after treatment with effective antimalarials.
Current experimental models are limited in trying to study this stage of malaria. But researchers at the Laboratori Europeu de Biologia Molecular – Barcelona (EMBL Barcelona) led by Maria Bernabeu have developed a 3D blood-brain barrier (BBB) model, which they have used to provide new insights into how the parasite manages to drive brain microvascular pathogenesis.
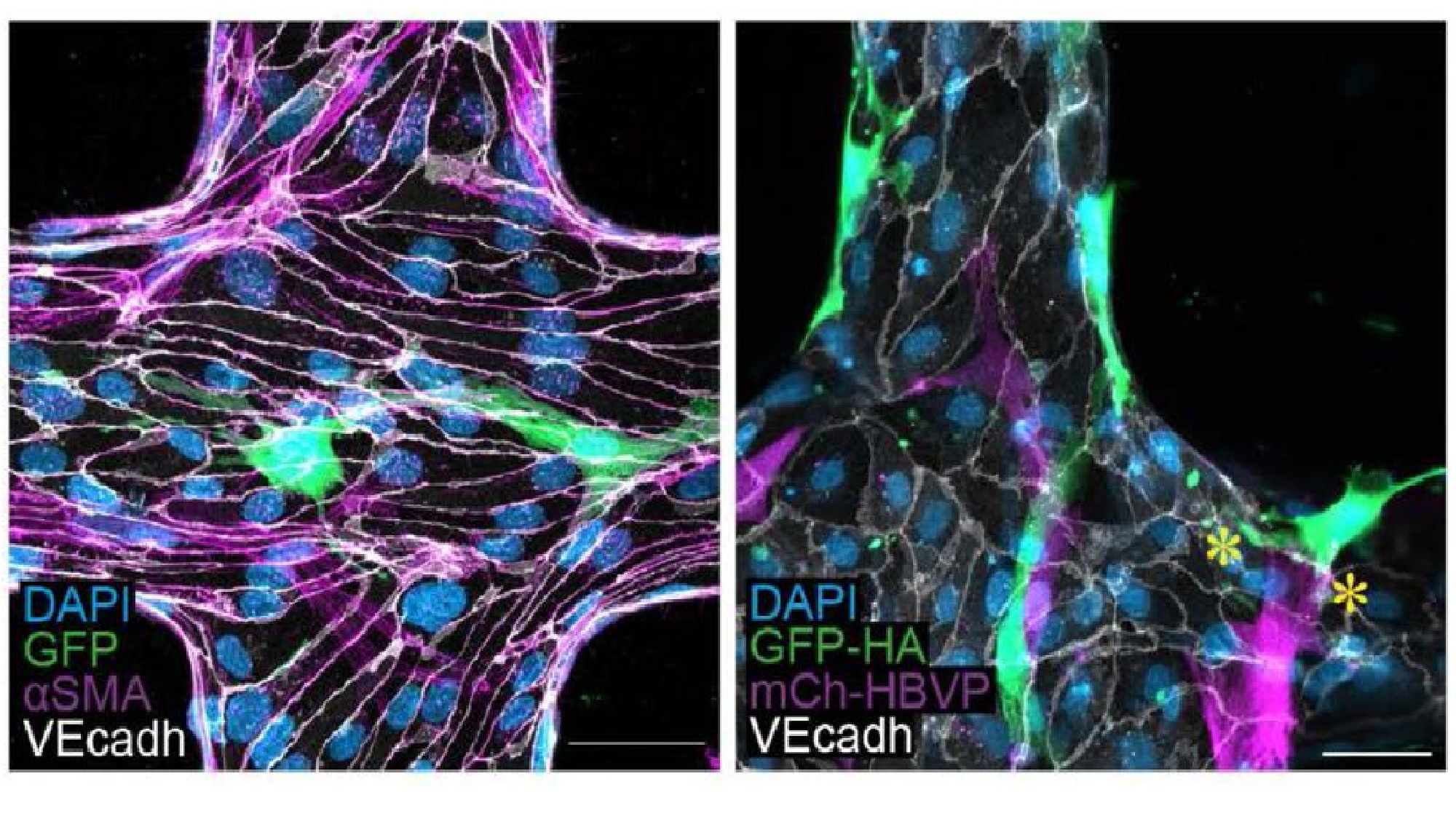
The image above shows z-projection images of their bioengineered 3D-BBB microvessel model, fabricated in a type I collagen hydrogel. In it, we can observe the spatial multicellular organization of the three types of cells that form the microvessel:
- The brain microvascular endothelial cells – which form the inside of blood vessels – are stained in white (marking the VE-cadherine protein).
- The primary astrocytes – the major type of nervous cells – are seen in green (via a GFP construct).
- The pericytes – a type of cells found on the walls of small blood vessels that help maintain their integrity – are labelled in pink.
In blue, we can see all cell nuclei.
Using this BBB model, the authors have shown in a recent preprint that the elements the malaria parasite eleases during egress (the phase in which the parasite leaves a host cell to go to another) are able to induce inflammation processes, disrupt the morphology of the endothelial junctions, and increase the permeability of the barrier. Based on these results, the authors suggest potential avenues for adjunctive therapies.