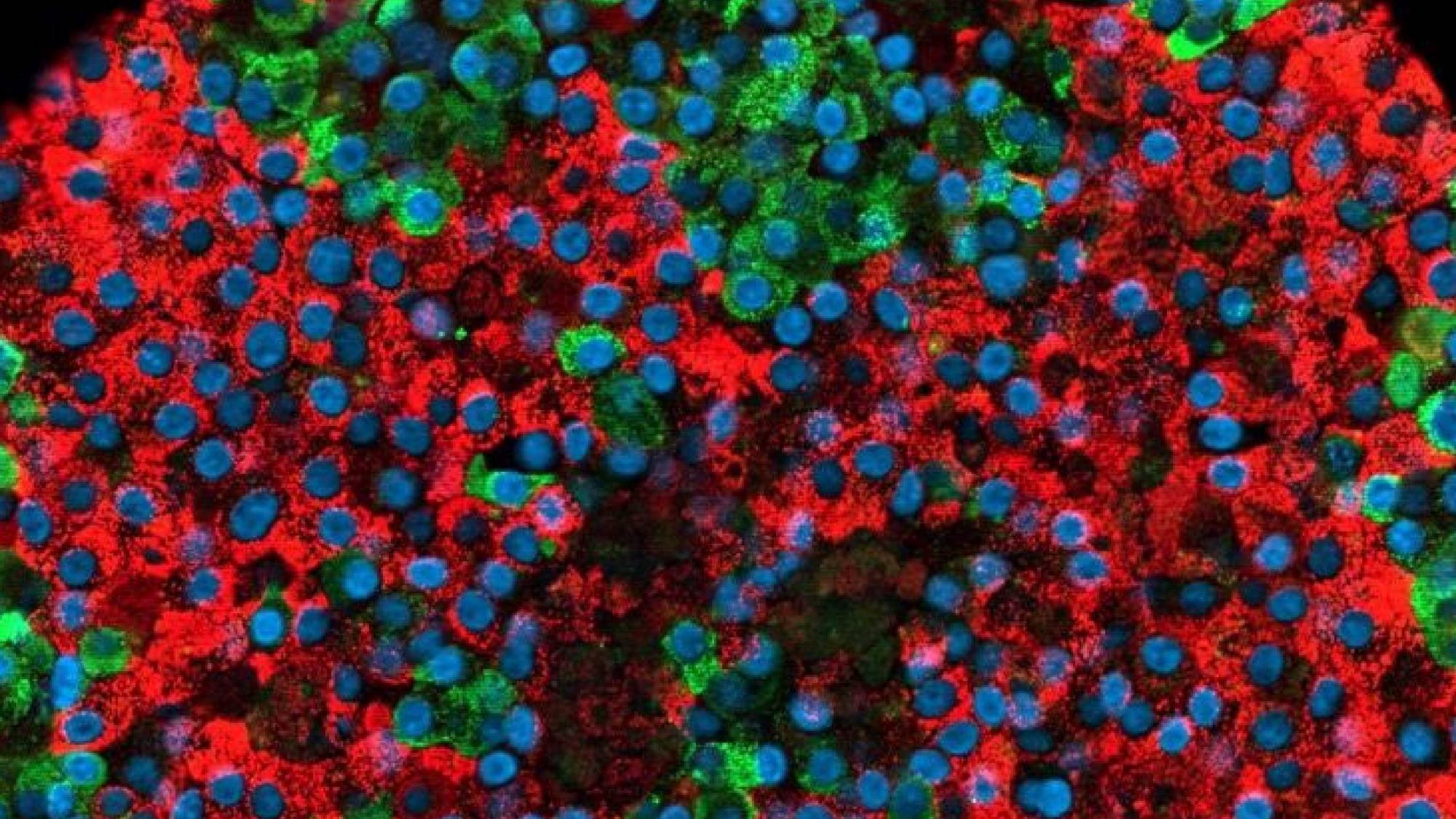
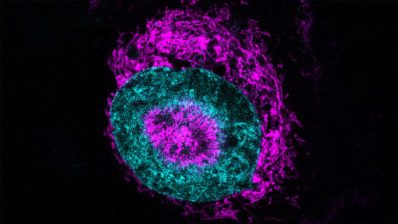
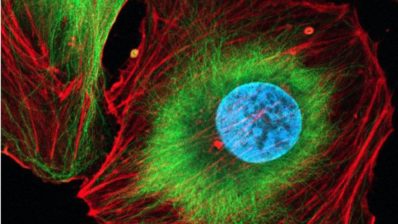
A team of scientists at the University of Helsinki in collaboration with the Centre for Genomic Regulation (CRG) has succeeded in creating functional pancreatic beta cells from stem cells for the first time, both in cell culture and in mice. Until now, research in this field had not been able to obtain fully mature pancreatic beta cells, so this finding represents a major breakthrough.
This research may pave the way for better treatment of type 1 diabetes. Type 1 diabetes involves the destruction of beta cells in the pancreas, which are responsible for producing insulin, the hormone that regulates blood sugar (glucose) levels. So far, transplants of beta cells isolated from the pancreas of donors have been used to treat the disease. However, this technique is complex and unusual.
This new study has succeeded in producing, from stem cells, cells that mimic in structure and function those of pancreatic islets. Moreover, the viability of these cells has been demonstrated both in cell culture and in mice, testing not only insulin secretion, but also glucose metabolism, ion channels and gene expression in these cells. In fact, in mice, transplantation of stem cell-derived beta cells has been shown to perform better in terms of glucose metabolism than transplantation of isolated donor beta cells.
This study, the most comprehensive to date, has succeeded in creating cells that mimic in structure and function those of pancreatic islets.
Moreover, says Diego Balboa, first author of the study and CRG researcher, “we can generate millions of these cells in the lab and use them for basic research: ask questions about the mechanisms of diabetes or the defects in genes or the cellular machinery that cause cells to fail”. “Unravelling the precise molecular causes of diabetes can help identify new therapeutic targets and explore the effects of new drugs”, he adds.
Balboa, D., Barsby, T., Lithovius, V. et al. Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells. Nat Biotechnol (2022). https://doi.org/10.1038/s41587-022-01219-z