From PhDs to Principal Investigators, we are very proud of our community of researchers at the Barcelona Biomedical Research Park (PRBB). Today we want to celebrate the recent achievements of two scientists: Anna Bigas, a group leader at Hospital del Mar Medical Research Institute (IMIM), and Akinola Akinbote, a PhD student at European Molecular Biology Laboratory – Barcelona (EMBL Barcelona).
Congratulations also to Ana Aldea and to Antonio García de Herreros, both from IMIM, for their recent appointments as members of national committees involved in the regulation of clinical practice and the evaluation of researchers, respectively.
Recognising female leaders
Anna Bigas, coordinator of the Stem cells and cancer Research Group of the IMIM has been chosen one of the Top 100 Women Leaders in Spain, in the category of Academics, researchers and thinkers.
This is a ranking created by Mujeres & Cia in 2011, an online magazine of reference among professional women that aims to make female talent visible and build a society based on equality.
Bigas, who is also the director of the Cancer CIBER (CIBERONC), is studying how stem cells that maintain different tissues in physiological conditions are generated and renewed. These processes have great similarities with those occurring in cancer, so her work focuses mainly on the hematopoietic system and the process of leukemia.
Highlighting African voices in STEM
Akinola Akinbote arrived to Kristina Haase’s lab at EMBL less than a year ago, but he’s already made an impression. He recently participated in the 2021 “Africans in STEM Symposium”, where he won an award for an outstanding flash talk on “Engineering 3D Human Cardiac Microvasculature on-chip to study coronary microvascular dysfunction“. We talk to him about this Symposium and what it means for him.
First of all Akinola, congratulations on your award! Can you tell us more about the Africans in STEM Symposium?
Thanks! Africans in STEM was founded at Cambridge University to “highlight scientific contributions by Africans in science, technology, engineering and mathematics, and to enable networks of support, sharing of ideas and collaboration”. They are also partnered with the Royal Society of Chemistry and Cambridge Africa. They organize annual symposiums as well as other short meetings to bring African scientists all over the world together, irrespective of their fields.
Was this the first time you participated?
Yes, this was the 3rd year they celebrated the Symposium, but it was my first time. There were between 60-100 people from all over the world; mostly from the United Kingdom, but also from other European countries and from African countries, as well as a few from the United States. There were also researchers at all levels, from undergraduates to professors, and both in academia and industry.
Why is it important to celebrate an African-specific symposium, and what did you get out of it?
I see it as an avenue to first of all meet people that look like me and that work in STEM, where we are still a minority, and then to connect with researchers that have a similar experience to mine, that come from a similar context. As an African – I come from Nigeria – I would like that my research helps find solutions that are relevant for my country, that the technology I am developing can be used in other countries, in our continent. And that is going to need people who have been there or who are based there and know what is needed. This Symposium gave me the opportunity to connect with several such people.
I would like the technologies I develop to be useful and relevant also for African countries; for that it is important to connect with people who are based there.
Akinola Akinbote (EMBL Barcelona)
And what technology are you developing?
In the Haase group we work on 3D vascularised in vitro tissues for disease modelling, drug development and regenerative medicine.
To overcome the limitations of current pre-clinical systems, such as 2D cell cultures (which are missing the 3D environment) or animal models (which can lead to species-specific differences), one needs 3D in vitro models. These can be organoids or microfluidic devices; in the Haase lab we are focusing on the latter.
These are 4-cm long polymeric devices made of PDMS, a kind of silicone, with a channel in the middle. We fill this channel with human primary cells mixed with a fibrin gel and culture them for 5-7 days; in these devices, given the appropriate environmental conditions, microvessels are formed! These vessels are particularly useful when formed in our devices because we can perfuse them with relevant solutes to understand the barrier properties of the vasculature, which is its main function in vivo, and we can also investigate how the barrier is altered with respect to changes in the environment. Kristina, the lab PI, developed these models earlier at MIT, where she used them to examine vascular health and dysfunction in cancer and in the placenta. In my case, I’m building on the previous models to engineer a cardiac-specific platform of in vitro vasculature.
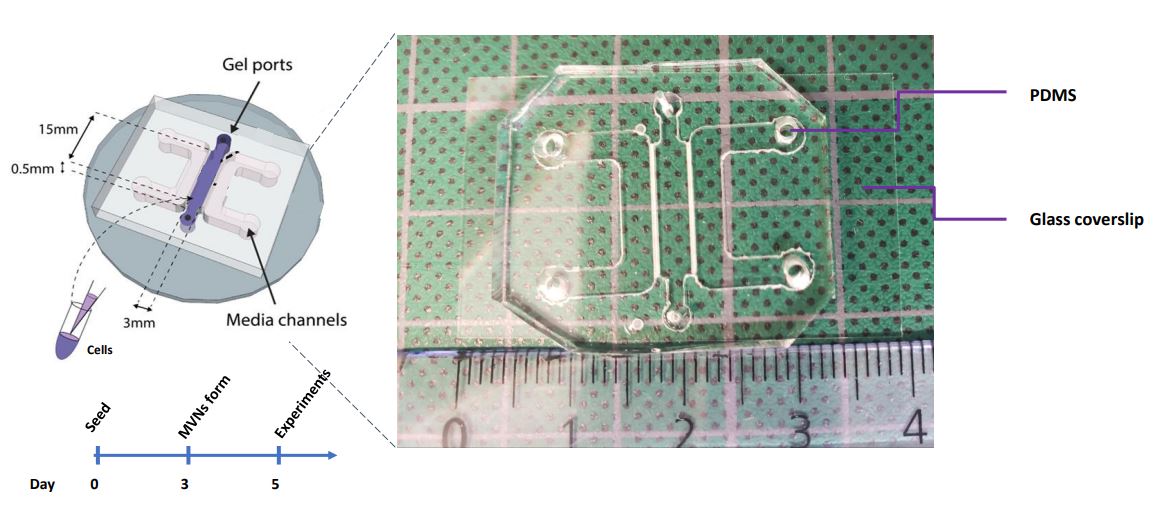
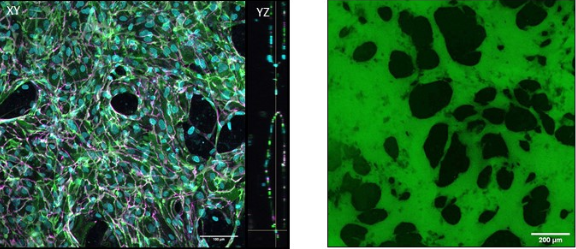
Why do we need cardiac-specific in vitro vessels?
Cardiovascular diseases cause nearly 18 million deaths annually, and recent evidence has pointed towards the heart’s smallest microvessels as major contributors to the pathological development of heart disease. Dysfunction of these small cardiac vessels is termed coronary microvascular dysfunction (CMD), and while there are a number of animal models being developed, there remains a need for human models to understand CMD.
We need to generate realistic heart-specific vessels in order to understand their development and how they become dysfunctional over time. Because blood vessels are characteristically specific to different tissues of the body we need to generate them from their tissue of origin. For example, cardiac endothelial cells need to withstand the constant beating of the heart and regulate nutrient and gas exchange in an energetically demanding tissue to support heart muscle contraction.
Building these vessels on-chip also allows us to manipulate the microenvironment, for instance by changing flow, hormonal conditions or oxygen concentration. Generating cardiac -specific vessels on-chip, in this way, will hopefully demonstrate key contributors to CMD and also allow us to examine the effect of relevant treatments. Given the understudied pathogenesis of CMD, along with sex and age-related contributing factors, it is really important to generate human cardiac microvessel models.







