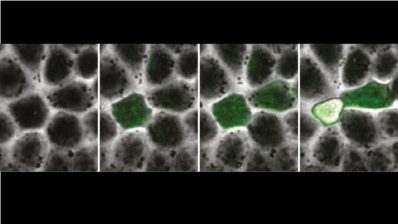
One of the key cellular processes in cell growth, division and communication with the environment is exocytosis: the active expulsion of encapsulated molecules to the outside. It is estimated that in one day, a single human cell can transport between 10.000 and 100.000 capsules to the cell membrane to release molecules outside the cell, such as the secretion of enzymes or hormones (as in the case of insulin) or other molecules that allow the cell to grow or move.
However, it was not until recently that a team led by the Department of Medicine and Life Sciences at Pompeu Fabra University (MELIS-UPF) identified the machinery that controls exocytosis. This discovery was made possible by combining optical and electron microscopes with artificial intelligence image analysis.
‘Despite being one of the largest nanomachines in the cell, its short lifespan and dynamism made it very difficult to capture’ Oriol Gallego (MELIS-UPF)
The research has shown that the machinery that enables exocytosis has a core formed by seven protein complexes, known as ExHOS. It is the flexible ring they form that allows these capsules to be attached to the cell membrane. ‘ExHOS has three control points and an unpacking mechanism that ensures that the delivery of goods into the cell continues at the right speed,’ explains Marta Puig-Tintó, a researcher at MELIS and one of the main authors of the article.
It is hoped that this discovery will have an impact on applied science in the future. This is because some pathogens, both plant-based, such as the fungus that infects rice, and human-based, such as SARS-CoV-2, HIV and Salmonella, use the exocytosis of host cells to infect them or weaken their defences.
Puig-Tintó, M; Ortiz, S; Meek, S; et al., Continuum architecture dynamics of vesicle tethering in exocytosis. Cell. Jan. 2026. DOI: 10.1016/j.cell.2025.11.038