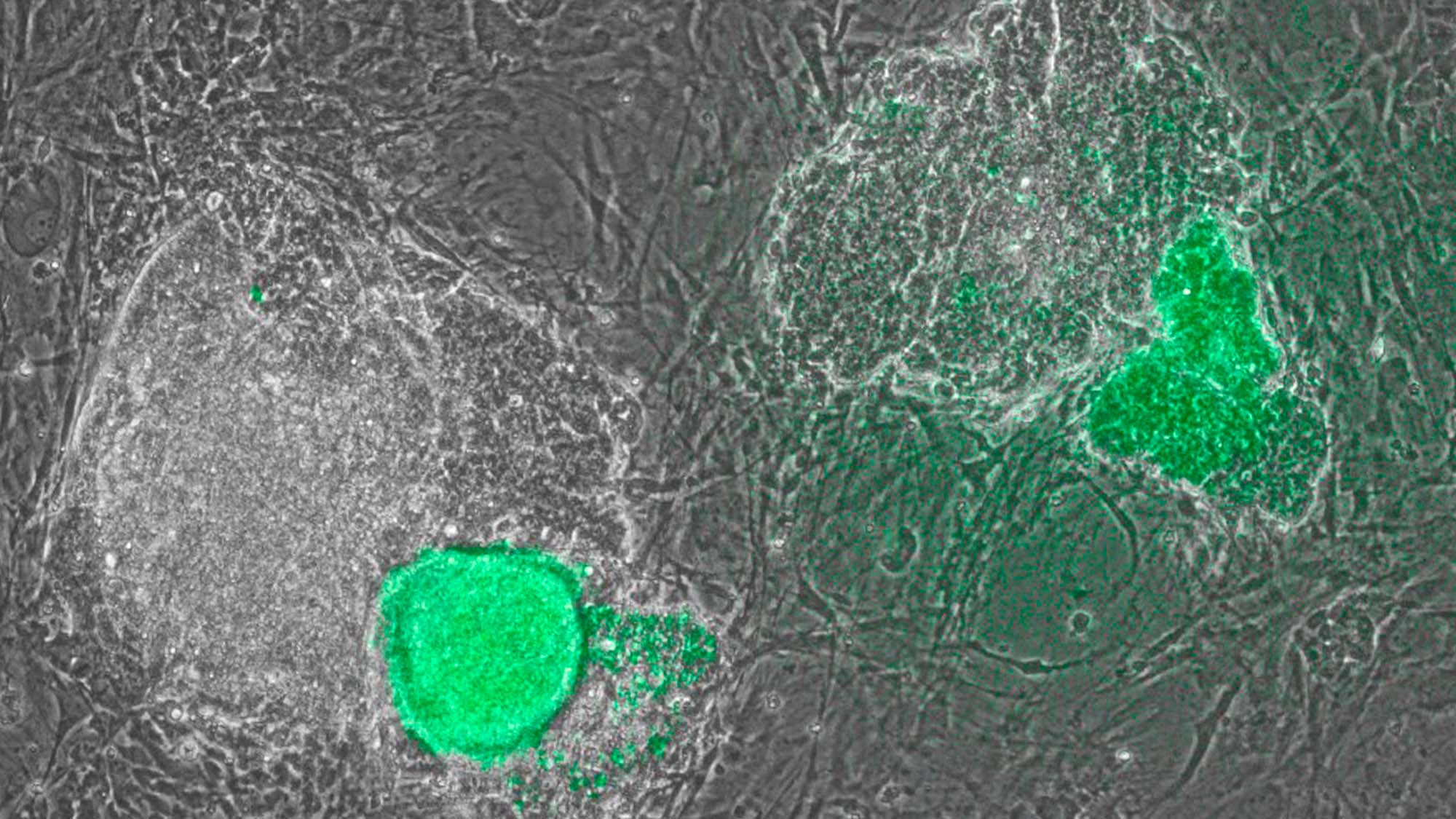
Induced pluripotent stem cells (iPSCs) are derived from adult cells that have been differentiated. This transformation into stem cells means that, once dedifferentiated, they can give rise to any other cell type. This is a complicated process, but one with great potential for medicine, particularly organ and tissue transplantation and other personalised treatments.
Historically, obtaining female iPSCs has been challenging. This is because one of the two X chromosomes is inactive in females to avoid duplication of genetic products. And to obtain high-quality stem cells, it is necessary to activate both X chromosomes during reprogramming.
Now, a collaboration between laboratories at the Centre for Genomic Regulation (CRG) and the Hospital del Mar Research Institute has found that interferon gamma (INFɣ), whose function is linked to the recruitment of the immune system during viral infections, could play a key role in obtaining iPSC cells from patients with two X chromosomes (mostly women, but also men who have an extra X chromosome due to Klinefelter’s syndrome, for example). They also observed that in a culture of mouse neuronal precursor cells, INFɣ reduced the time to obtain iPSCs by one day.
“INFɣ helps cells respond to viral infection by opening up DNA and rapidly activating gene expression. One possible explanation for its role in reprogramming is that by opening the DNA like a clam, certain genes are exposed, facilitating their reprogramming and accelerating the cell’s transformation into a stem cell”
Mercedes Barrero, first author (CRG)
“The addition of INFɣ to mouse cells made the reactivation of the X chromosome twice as efficient. Although the resulting iPSCs were of the same quality, we suspect that in humans, INFɣ is likely to be a key factor in improving the quality of female cell lines,” explains Bernhard Payer, principal investigator of the study and group leader at the CRG. Payer also emphasises that this finding paves the way for the production of high-quality female iPSCs, ensuring the promise of personalized medicine for everyone”.
Mercedes Barrero et al. The interferon γ pathway enhances pluripotency and X-chromosome reactivation in iPSC reprogramming. Sci. Adv.10, eadj8862(2024). DOI: 10.1126/sciadv.adj8862