The conditions in which an individual enters the Intensive Care Unit (ICU) of a hospital are critical. Patients are usually in such a state that they are unable to breathe on their own. If we want to save their life, it is essential they undergo mechanical ventilation. However, its use, limited to keeping the patient alive, is not free of risks.
A recent study conducted by doctors from the Hospital del Mar and researchers from the Hospital del Mar Medical Research Institute (IMIM) highlighted that promoting mechanical ventilation strategies that allow ICU patients’ respiratory muscles to work, decreases the damage they suffer and could help in their recovery.
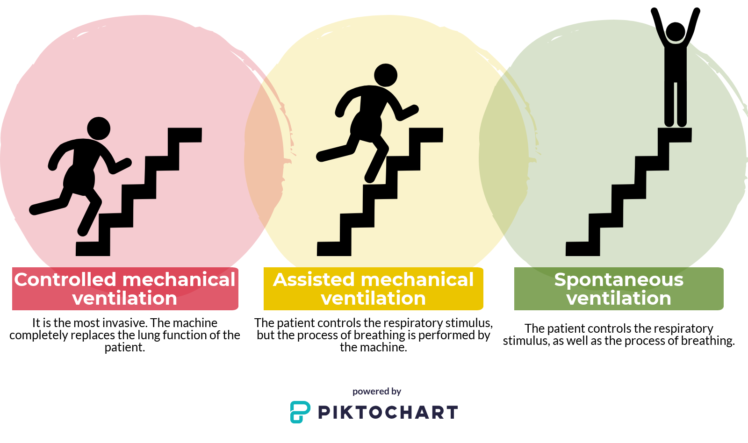
There are three different types of mechanical ventilation:
- Controlled mechanical ventilation: it is the most invasive. The machine completely replaces the patient’s respiratory function.
- Assisted mechanical ventilation: the patient controls the respiratory stimulus, but the process of breathing is performed by the machine.
- Spontaneous ventilation: the patient controls the respiratory stimulus, as well as the process of breathing.
Doctors at the ICU will try to withdraw artificial ventilation as soon as possible so that the patient starts breathing again as soon as possible, in order to avoid some of the problems that may arise from mechanical ventilation.
We interview Dr Judith Marín, first author of the article.

Why is it important to study muscular dysfunction in critical patients?
During many years, the main objective has been – understandably! – saving the patients lives. This led us to be very invasive, neglecting important aspects such as the muscle dysfunction that they suffer after a prolonged stay at the ICU. Nowadays, we know that this muscle dysfunction leads to a worse quality of life of the survivors.
This is why it is important to study this field, as well as the implementation of the tools we already have to evaluate and treat muscle dysfunction to avoid that it conditions the daily life of the patients once they leave the ICU.
What is meant by mechanical ventilation?
When patients suffer from severe respiratory failure or are at a level of consciousness that does not allow them to breathe on their own, we must use devices that supplement their respiratory function. These devices can be non-invasive or invasive. Our study has focused on the invasive mechanical ventilation.
Regarding mechanical ventilation, patients are intubated and connected to a machine. Depending on how we operate the machine, we will talk about controlled, assisted or spontaneous mechanical ventilation.
Depending on the type of mechanical ventilation, a different degree of dysfunction will be induced in the patient. Controlled mechanical ventilation would be the most damaging type.
What have you found regarding the types of mechanical ventilation?
This is the first study in humans that shows that, depending on the type of mechanical ventilation we use when assisting patients’ breathing, we will be inducing a different degree of dysfunction. The more we let the respiratory muscles of the patient work, the better.
In this line, we have seen that the most damaging type of assisted breathing is controlled mechanical ventilation and, therefore, we should avoid it whenever possible. If we let the patient’s respiratory muscles work as soon as possible, we will shorten the period of mechanical ventilation and, consequently, their stay at the ICU.
How have you shown that controlled mechanical ventilation is the most damaging?
In the literature, the studies that relate the mechanisms of mechanical ventilation with the damage of the respiratory musculature have been carried out from samples of organ donors. Diaphragm biopsies of ICU patients who are subjected to mechanical ventilation are not usually used because, due to the critical state of these individuals, the procedure is complicated and not recommended.
The novelty of our study is the incorporation of a new type of donor: the Maastricht III. Up to now, only brain-dead donors were studied. That is, donors who had suffered severe damage at the level of the Central Nervous System and, therefore, could not exercise their diaphragm. This type of donors can undergo controlled mechanical ventilation.
Donors in controlled asystole (Maastricht III) have also suffered damage at the level of the Central Nervous System but, unlike the former, they have not reached brain death, so they remain in a coma or vegetative state. These individuals can have respiratory stimuli and, therefore, exercise their diaphragm. Maastricht III donors can undergo assisted mechanical ventilation.
In the study, it has been shown that, after 100 hours undergoing mechanical ventilation and without exercising the respiratory muscles, donnors start to show alterations in their respiratory musculature. However, it is known that fewer hours are already harmful.
After studying the respiratory musculature of both types of donors we have seen how, in comparison with the Maastricht III, brain-dead patients had smaller muscle fibers and histopathological alterations in their respiratory muscles.
Since obtaining biopsies in critical patients is complicated, is there another way to analyse the diaphragm of these individuals?
Yes, through diaphragmatic ultrasound. Actually, we see that the thickness of muscle fibers observed by ultrasound correlates very well with what we observe in histological studies of donor samples.
We want these ultrasounds to become systematic in order to follow the course of diaphragm atrophy. We think this is the work that will let us demonstrate that, the shorter the periods of controlled mechanical ventilation, the shorter the recovery times.
How long will it take for the patient to recover?
It depends a lot on the patient. What we have seen is that, if a patient tolerates spontaneous ventilation, it is necessary to change from controlled mechanical ventilation to spontaneous ventilation as soon as possible; in animal studies, it has been shown that diaphragmatic dysfunction associated with mechanical ventilation occurs in 6-12 h.
Diaphragm dysfunction occurs in only a few hours of being subjected to mechanical ventilation.
From now on, and thanks to the evidence provided, do you think you will reduce the number of patients undergoing controlled mechanical ventilation?
What we can reduce is not so much the number of patients who need controlled mechanical ventilation, but the time that these patients are subjected to this type of ventilation. Many times we see that the time of controlled mechanical ventilation required by the pathology is prolonged by other factors, such as excessive sedation. That is why new protocols must be established. Increasingly, we have more tools and drugs that allow patients to be awake and calm to tolerate mechanisms of mechanical ventilation. Work must be done on the early mobilization of these individuals to make their muscles work, as well as on improving their nutritional status.