Glycolysis is traditionally known as a basic power-producing process in cells – the basic metabolism that converts sugars into energy. Now, teams of researchers at the European Molecular Biology Laboratory – Barcelona (EMBL Barcelona) and the Max Planck Institute CBG in Dresden have independently found that it plays a much more active role in embryonic development than previously thought.
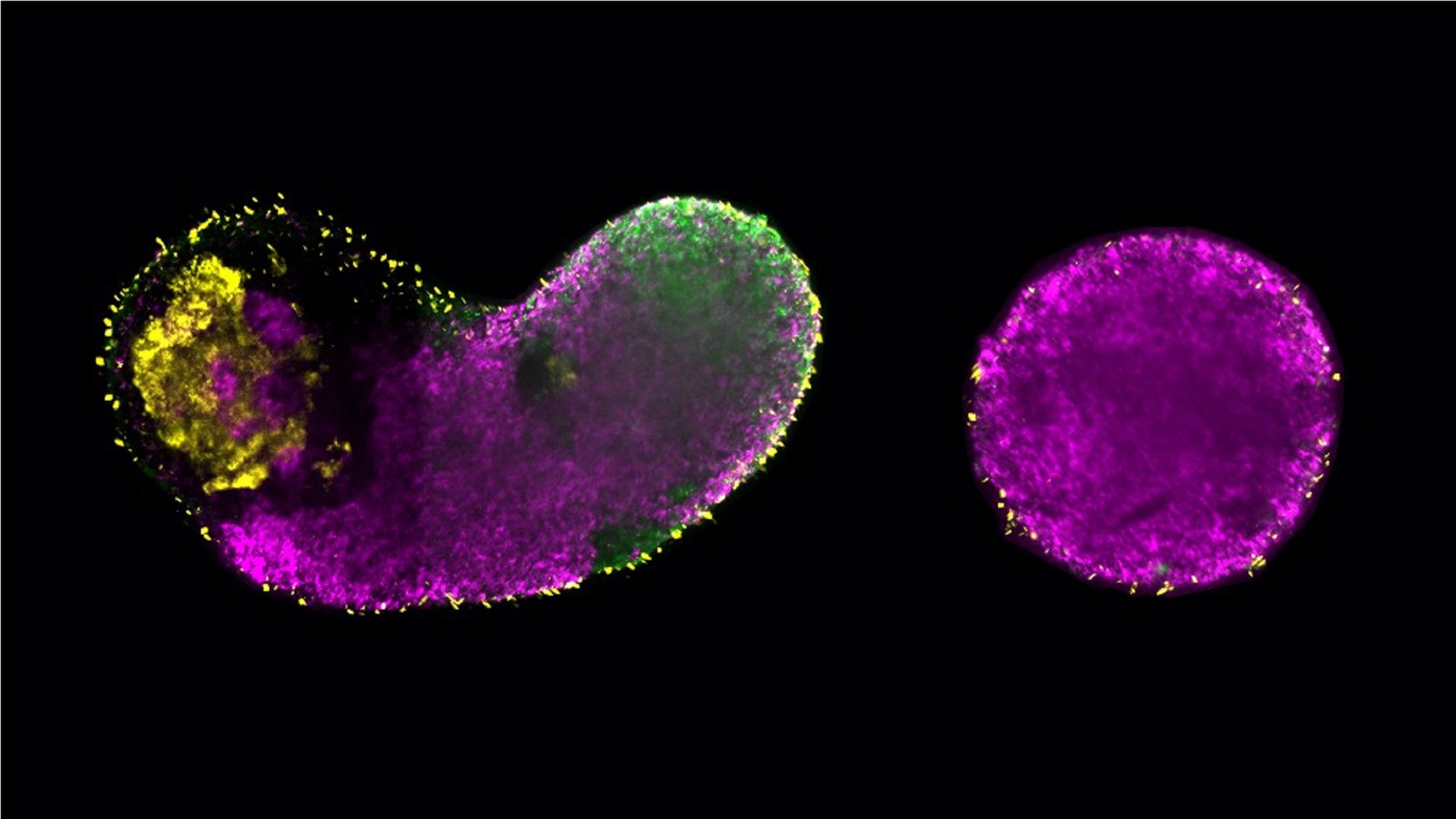
The EMBL Barcelona scientists, from Vikas Trivedi’s lab, demonstrated that glycolysis not only fuels cell growth but also directly influences early cell fate decisions by regulating crucial signalling pathways like Wnt, Nodal, and Fgf. Using stem-cell-based embryo models (generated from mouse and human embryonic stem cells), they showed that manipulating glycolysis impacts whether cells develop into mesoderm and endoderm (key tissue types for muscles, blood, and organs) or default to ectoderm (nervous system). Intriguingly, with glycolysis blocked, artificially boosting the signalling pathways could restore normal development, but not glycolysis, revealing glycolysis’s dual role: metabolic and instructive.
“The results highlight the crucial role of metabolism as an upstream regulator of specific signalling pathways that influence cellular decisions”
Kristina Stapornwongkul, (EMBL Barcelona. IMBA)
An interesting thing is that these two roles are uncoupled, says Kristina Stapornwongkul, a postdoc in Trivedi’s lab who is now starting her own independet group leader position at IMBA, Vienna, Austria. “From an evolutionary perspective, this is exciting because metabolism predates signalling”, adds Trivedi. “Even single-cell organisms rely on metabolism, while signalling emerged later in evolution. This has sparked my curiosity about the role of metabolism in the origin of multicellularity”.
“This study marks the beginning of an exciting new direction for my group”
Vikas Trivedi (EMBL Barcelona)
The Dresden team, led by Jesse Veenvliet, used artificial intelligence to reach similar conclusions about the role of glycolysis in steering developmental decisions in the embryo.
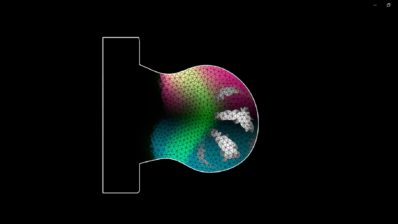
They started by addressing the existing variability in trunk-like structures, a stem-cell-based model of embryonic trunk development. Even under identical conditions, some models resembled natural embryos better than others. The researchers found that an early metabolic preference for glycolysis, versus oxidative phosphorylation (another of the cell’s way of creating energy), predicts better developmental outcomes.
Through machine learning and imaging, they identified early metabolic markers that forecast developmental success. They also showed that boosting glycolysis with drugs improves the consistency and quality of these models. These findings open new possibilities for more reproducible stem-cell-based models, crucial for disease modeling, drug testing, and basic developmental biology, while minimizing animal use.
Together, these studies mark a shift in how scientists understand the relationship between metabolism and development. Rather than being a background process, metabolism—particularly glycolysis—actively steers the formation of body structures from the very first steps of life.
Barcelona study: Glycolytic activity instructs germ layer proportions through regulation of Nodal and Wnt signaling, Cell Stem Cell (2025). Stapornwongkul K. et al. Cell Stem Cell 16/04/2025
Desden study: Integrated molecular-phenotypic profiling reveals metabolic control of morphological variation in a stem-cell-based embryo model, Villaronga-Luque A., Savill R. G., et al. Cell Stem Cell 16/04/2025. https://doi.org/10.1016/j.stem.2025.03.012